Welfare considerations for farmed shrimp
Shrimp welfare research at Rethink Priorities
Rethink Priorities’ Shrimp Welfare Sequence is a series that addresses whether and how best to protect the welfare of shrimp. At any time, more shrimp are alive on farms than any other group of farmed animals. But is the large size of the industry an issue? After all, farmers might treat shrimp well, and shrimp may not even be sentient. In upcoming reports, we explore a cause for concern: a large percentage of these individuals die before they are old enough to be slaughtered. After describing farming practices that help explain high mortality rates, we use a quantitative model to explain why the most urgent issues are not necessarily the ones that have attracted the most attention.
For all queries, please contact hannah@rethinkpriorities.org.
Executive summary
This is the second report in Rethink Priorities’ Shrimp Welfare Sequence, a series that addresses whether and how to best protect the welfare of shrimp. While there is uncertainty about whether shrimp are sentient (Crump et al., 2022), the Animal Sentience Precautionary Principle suggests that we should not postpone helping shrimp if there are “threats of serious, negative animal welfare outcomes” (Birch, 2017, p. 3). To better understand if such outcomes do threaten shrimp, our first report estimated the scale of shrimp farming. We estimated that approximately 230 billion shrimp are alive on farms at any one time, more than any other farmed animal (Waldhorn & Autric, 2023).
However, just because the scale of shrimp farming is so comparatively large does not necessarily mean that it causes more suffering than other sectors. To understand if shrimp experience negative welfare, we reviewed academic and gray literature and consulted several shrimp aquaculture experts to describe the welfare threats a farmed shrimp may face from hatching to slaughter.
Key takeaways include:
- If they are sentient, shrimp face several welfare threats. Diseases preoccupy farmers the most, but they are often a downstream consequence of other issues, such as poor water quality. Some issues affect all shrimp for most of their lives, such as a lack of substrate, whereas others only affect subpopulations, such as eyestalk ablation of female breeders. Shrimp are particularly vulnerable during harvest and slaughter, where they endure oxidative stress and can be crushed by other shrimp.
- The prevalence of welfare threats varies widely across farms with different production practices. As farm intensity increases, the rearing environment resembles that of a wild shrimp less and less. Welfare issues on so-called "extensive" farms result from an inability to address threats to shrimp welfare. In contrast, "intensive" farms can mitigate problems that are common on extensive farms but must confront the novel issues introduced by high stocking densities. Many solutions cause problems of their own, and some of the issues are likely not solvable without lowering stocking densities.
- We have several knowledge gaps due to the limitations of current research. In particular:
- We know very little about shrimp behavior and preferences. For example, we are unsure whether shrimp can comfortably crowd, so we cannot easily infer how bad high stocking densities are for their welfare.
- There are few surveys of shrimp farming practices and those that exist have several limitations. The heterogeneity of shrimp farming means it is difficult to draw conclusions about the industry from existing surveys.
Box 1: Shrimp aquaculture terminology
The terms ‘shrimp’ and ‘prawn’ are often used interchangeably. The two terms do not reliably track any phylogenetic differences between species. Here, we use only the term “shrimp”, covering both shrimp and prawns. Note that members of the family Artemiidae are commonly referred to as "brine shrimp" but are not decapods and so are beyond the present scope. We opt for the use of Penaues vannamei over Litopenaeus vannamei (to which this species is often referred), due to recognition of the former but not the latter nomenclature by ITIS, WorMS, and the Food and Agriculture Organization of the United Nations (FAO) ASFIS List of Species for Fishery Statistics Purposes. The shrimp farming industry uses many terms usually associated with agriculture—for example, ‘crops’ for a group of shrimp reared together, ‘seed’ for the first shrimp stocked into a pond, and ‘harvest’ for collecting and slaughtering shrimp. For clarity, we broadly conform to this terminology. Although we acknowledge animal welfare advocates may prefer terminology that does not euphemize or sanitize the experience of farmed shrimp, here we favor ensuring readability for a wide audience. |
|---|
Introduction
Should farmed shrimp receive protection as a precautionary measure?
Although calls to improve the welfare of farmed animals have increased in recent decades (e.g., European Commission, 2023; Sinclair et al., 2022), the needs of farmed shrimp (see Box 1) in particular have only barely begun to receive attention (Pedrazzani et al., 2023). Only sentient animals can suffer, so this apparent oversight may be due to uncertainty about whether shrimp are sentient. Regrettably, existing empirical evidence does not provide a decisive answer (see Box 2). In the interim, there is a need for guidance on how much attention to give shrimp welfare. The Animal Sentience Precautionary Principle states that when there is a risk of "serious, negative animal welfare outcomes, lack of full scientific certainty as to the sentience of the animals in question shall not be used as a reason for postponing cost-effective measures to prevent those outcomes" (Birch, 2017, p. 3; emphasis ours).
The number of individuals affected is one measure of how serious negative animal welfare outcomes would be (Birch, 2017, p. 4). On this metric, shrimp aquaculture certainly qualifies as serious: the first report of the Shrimp Welfare Sequence estimates that there are more shrimp alive on farms at any time than any other farmed animal (~230 billion; Waldhorn & Autric, 2023). But how often do farmed shrimp face the negative outcomes required to invoke the Animal Sentience Precautionary Principle? To find out, this report describes the rearing conditions and slaughter of farmed decapod shrimp, focusing on the most commonly farmed marine (viz., Penaeus vannamei and Penaeus monodon) and freshwater species (viz., Macrobrachium rosenbergii).
Box 2: Shrimp sentience researchSentience refers to the capacity for valenced (i.e., positive or negative) experiences. Experiences cannot be directly observed, and so evidence of shrimp sentience can only be inferred based on physiological and behavioral evidence. Researchers have reported three types of empirical evidence that shrimp can feel pain:
Not only is the little extant evidence relevant to shrimp sentience ambiguous, but it also primarily focuses on Caridean shrimp, a different evolutionary group than penaeids who make up the majority of farmed shrimp (Crump et al., 2022; see Figure 3 below). However, a lack of relevant research is not itself evidence against shrimp sentience. Going forward, the most informative tests would involve observing whether shrimp can make trade-offs between novel sets of opportunities and threats (for more detail, see Farnsworth and Elwood, 2023). |
|---|
Measuring shrimp welfare
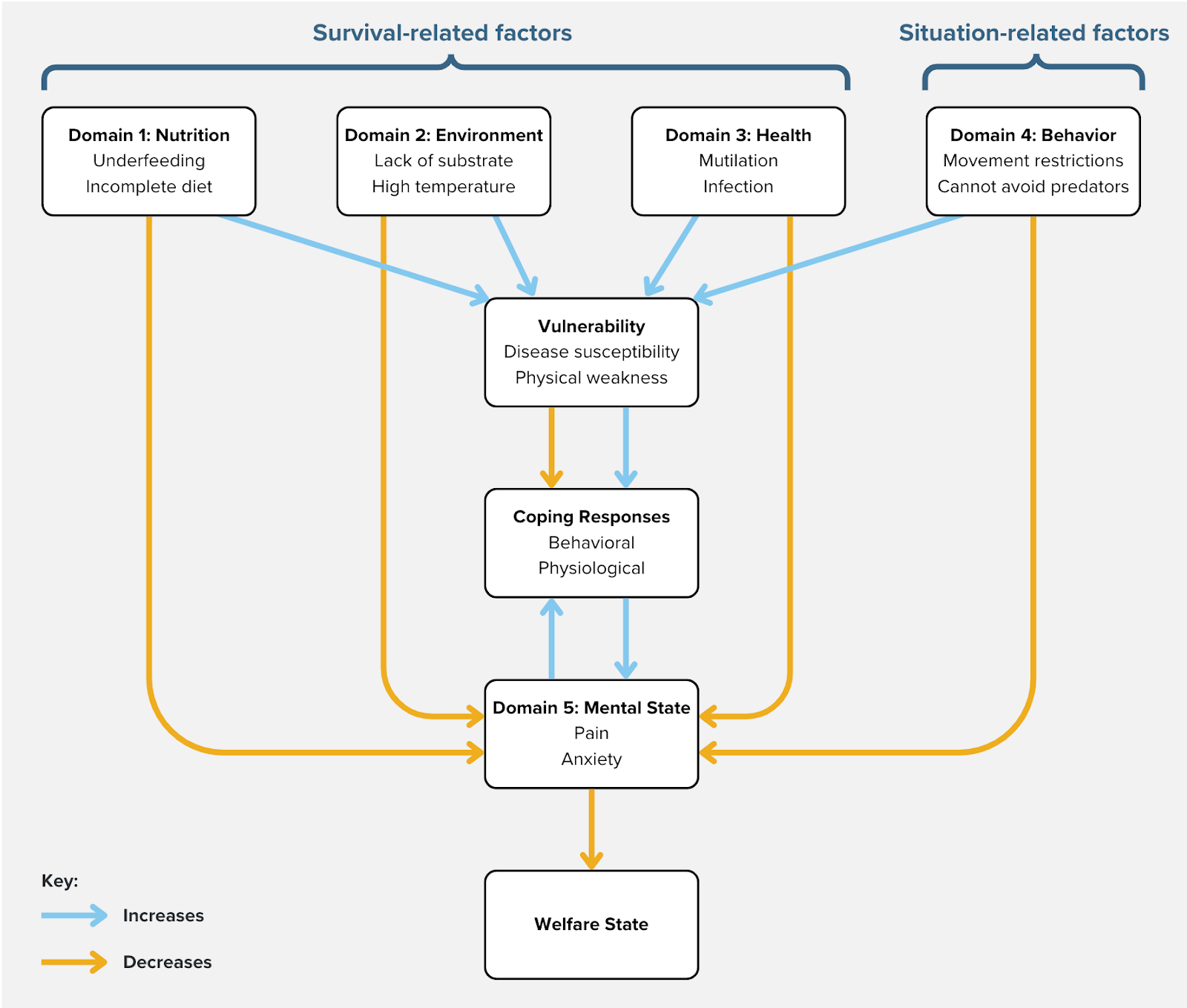
We embrace an affect-based definition of welfare that identifies an animal's welfare with the valence, duration, and intensity of its experiences. To identify proxies for valenced experience, we adopt the Five Domains framework (Mellor et al., 2020; see Figure 1), which was originally applied to terrestrial vertebrates but has recently been extended to aquatic invertebrates (Pedrazzani et al., 2023; Perkins, 2021). Domain 1 is Nutrition (e.g., restricted feed intake reduces welfare, a balanced diet increases it), Domain 2 is Environment (e.g., overcrowding reduces welfare, a clean space increases it), Domain 3 is Health (e.g., mutilations reduce welfare, high immune capacity increases it), and Domain 4 is Behavior (e.g., a barren environment reduces welfare, an absence of predators increases it). The first four domains do not themselves reflect good or bad welfare, but instead influence welfare via Domain 5, Mental State (e.g., pain is a manifestation of negative welfare, comfort is a positive welfare state; Mellor & Beausoleil, 2015, p. 242).
Figure 1: Adaptation of Five Domains framework based on Mellor and Beausoleil (2015). Only negative states are depicted here given this report's focus on threats. Vulnerability is added to distinguish between threats that directly and indirectly reduce welfare.
Threats in the first four domains will clearly affect biological fitness, but why would they cause negative experiences? Indeed, some genuine welfare threats probably only influence experience indirectly (Broom, 1991). For example, poor immune health may not cause negative experiences on its own, but it would still constitute a welfare threat if it exacerbated negative experiences during an infection. However, in many cases, it is plausible that valenced experience mediates the perception of threats and physiological and behavioral responses that mitigate the threats. In particular, Domains 1 through 3 are "survival-related factors" that arouse emotions to motivate behavior that would safeguard fitness (e.g., thirst motivates the search for water). Meanwhile, Domain 4 represents "situation-related factors" that arouse negative emotions in preparation to respond (e.g., the presence of predators causes anxiety). Also, environments that thwart fitness-enhancing behaviors will arouse negative emotions in an attempt to change the situation (e.g., the inability to move freely causes frustration). Although these emotional responses evolved in ancestral environments, they may still occur even when they are irrelevant to survival in captivity. For example, social species may experience reduced welfare when isolated on farms, even though conspecifics have no ability to enhance their fitness in that context.
The Five Domains model has been updated to account for positive welfare (Mellor & Beausoleil, 2015, pp. 244–245). However, many theorized determinants of positive welfare result from successfully confronting welfare threats (e.g., the cessation of pain causes relief) or being free from them in the first place (e.g., the absence of predators causes calmness). Furthermore, because our goal is to determine whether the precautionary principle is applicable to shrimp aquaculture, we focus here on negative threats only. We signpost at the titles of sections which survival- and situation-related factors are implicated in the discussed welfare threats.
Background on shrimp farming
The majority of shrimp used as food (in tonnes) now comes from farmed shrimp, as opposed to wild-caught shrimp (Figure 1). Just three species make up the majority of farmed individuals—P. vannamei (family: Penaeidae), P. monodon (family: Penaeidae), and M. rosenbergii (family: Palaemonidae; infraorder: Caridea). Due to its fast growth rate and high tolerance for a range of environmental conditions, P. vannamei alone accounts for approximately 82% of global shrimp aquaculture production (Waldhorn & Autric, 2023). P. vannamei and P. monodon are marine species farmed using similar approaches, while M. rosenbergii is a freshwater species reared under different conditions (Figure 3).
Figure 2: Tonnes of shrimp produced globally from 1950 to 2021 from aquaculture (black) and wild capture (orange), along with contributions to aquaculture tonnage by P. vannamei (dashed blue), P. monodon (dashed green), and M. rosenbergii (dashed pink). Based on FAO (2023a; b).
Figure 3: Partial phylogeny of decapod shrimp. Taxonomic rank is in yellow, and in blue are the taxa we focus on throughout the report. Based on Bauer (2004, p. 206) and Wolfe et al. (2019).
The shrimp farming cycle
The shrimp life course is divided into distinct stages: eggs, larval,1 postlarval, juvenile–subadult, and adult (Figure 4b). Adults are only used as breeders (known as broodstock; Figure 4b) and most shrimp are harvested at the juvenile–subadult stage before they become sexually mature. In the farming process, shrimp of different life stages are moved to corresponding rearing environments (Figure 4c). Each rearing stage in Figure 4 represents a different body of water. The rearing environments that eggs, larvae, postlarvae, and broodstock are kept in are commonly referred to as tanks, whereas juvenile–subadults are generally referred to as being in ponds (see FAO, 2009a)—we, therefore, most commonly use this terminology throughout the report but note that different shrimp farms may vary. Other types of rearing environments include raceways.2
Figure 4: Conceptual diagram of a) the shrimp farming process different facilities cover; (b) the life stages of farmed shrimp; (c) the associated rearing stages of the farming process and (d) the inputs of different types of shrimp into the shrimp farming systems. Dashed yellow lines represent when shrimp at different life stages are moved to different rearing stages. Based on FAO (2009a; 2009b; 2023c).
To begin the farming cycle (Figure 4d), the industry predominantly relies on domesticated broodstock to supply shrimp farms3 due to the disease risks from wild-caught shrimp (seeDiseases section). There are specialized broodstock facilities dedicated to producing and supplying adult shrimp.4 Some small farms may use wild “seed” shrimp to stock their ponds. Broodstock are matured in maturation tanks, often using eyestalk ablation (see Eyestalk ablation section), and they lay their eggs in spawning tanks. The eggs are moved to hatching tanks for hatching. The resultant larvae are moved to larval-rearing tanks until they reach the postlarval stage. Postlarvae may be moved straight to ongrowing ponds or first to a nursery to develop more.5 Shrimp grow in ongrowing ponds until they reach the juvenile–subadult stage, when they are harvested. Shrimp are reared for a total of three to six months (Prangnell et al., 2019, p. 19) and the majority of this time—around two to five months—is spent in the ongrowing rearing facility.
In the wild, penaeid shrimp (those from the Penaeidae family; see Figure 3) spawn their eggs in the open ocean, where the eggs hatch into larvae. After about ten days, they reach the postlarval stage and migrate to inshore, brackish waters, developing a benthic lifestyle by the fifth day of this stage (i.e., primarily crawling instead of swimming; Benchmark Genetics, 2022; FAO, 2009a; Prangnell et al., 2019, p. 19). They return to the ocean when they reach the adult stage. Conversely, M. rosenbergii hatch their eggs in brackish water, where the larvae develop, before returning to freshwater at the postlarval stage (FAO, 2009b). The water used in different rearing stages therefore has to be adjusted accordingly for the species and shrimp life stage it hosts.
Shrimp feed also has to change over their life course, as they grow larger and their digestive tracts become more complex (Reis et al., 2022, p.357). Larval shrimp are small and planktonic, so they are fed algae and live Artemia (brine shrimp) larvae.6 By the ongrowing stage, they become benthic, opportunistic feeders and are generally considered omnivores (though P. monodon is more carnivorous and predatory than P. vannamei; FAO, 2023c; Prangnell et al., 2019, p. 24), feeding on algae, detritus, other small crustaceans, and polychaetes, amongst others, in the wild (Bardera et al., 2022, p. 337; Kungvankij et al., 1986a). On farms, they are fed artificial feeds or the natural productivity of the pond sustains them (Bauer, 2023, p. 628; FAO, 2009a).
Shrimp grow by molting their exoskeleton, a process demanding great energy expenditure (Penn et al., 2001, p. 699). They shed their old exoskeleton and then grow considerably before developing a new one. Therefore, individuals increase in weight and size in abrupt stages (New, 2002, pp. 8–9). Between a molt and the development of a new exoskeleton is a period of time where a shrimp has no hard exoskeleton, and is therefore particularly vulnerable to outside threats like disease or damage from conflict.
The various structures related to different life stages may be present at different shrimp farming facilities in different combinations (Figure 4a). For example, a fully integrated farm may have its own broodstock facility, hatchery, and ongrowing system (e.g., see Sun Shrimp). Or, a farm may have an on-site hatchery and ongrowing ponds. Alternatively, a large-scale hatchery may purchase broodstock from a specialized facility and supply postlarvae to stand-alone ongrowing farms. A small-scale hatchery may purchase fertilized eggs or larvae from a larger hatchery and then rear them to the postlarval stage before supplying them to an ongrowing facility.
Even on fully integrated farms shrimp are handled so they can be transferred between tanks. Shrimp can be transferred using pumps, draining to a different pond at a lower elevation, placed in transport tanks and carried, or transferred via nets (Villalón, 1991). Within the same tank, shrimp may be handled briefly to check for things like disease and growth status.
In other cases, shrimp are transported alive between facilities, which are sometimes in different countries or even continents. For shorter distances, water-based methods are used to transport shrimp by car or transfer by foot. Shrimp are placed in boxes with a fine mesh bottom and lid. These boxes, in turn, are placed in tanks with circulating water and controlled temperature (Wickins & Lee, 2002, p. 137). Alternatively, they are transported in polyethylene bags with water. Commonly, oxygen is provided and the water temperature is controlled using ice packs and air-conditioned trucks (Olin & Fast, 1992, p. 313; Wickins & Lee, 2002, p. 144). For longer distances, waterless methods are used to transport shrimp by car or plane. Farmers first reduce the water temperature to reduce the shrimp's metabolic rate and prevent cannibalism (Kuhn & Taylor, 2017). They are then placed on chilled wood shavings or hemp, which are placed in hermetically sealed containers enriched with pressurized oxygen and kept cool with ice packs. Some shrimp are transported live at the end of production as well. In some countries, consumers are willing to pay a price premium to purchase shrimp alive (Guan et al., 2021). Some shrimp are also sold live to be used as fishing bait (Florida Shore Fishing, 2014).
Once shrimp have reached market size, they are harvested and (if not being sold live) slaughtered. Some farmers use partial harvesting, where some shrimp are harvested during the farming cycle to improve cash flow or create more room in the pond for other shrimp to grow larger (Estrada-Pérez et al., 2016; Yu et al., 2009). Total harvesting, on the other hand, involves removing all shrimp at one time at the end of the production cycle.
A variety of trapping or collection methods are used in both types of harvest. Most involve nets of varying levels of sophistication. Cast nets are weighted and capture everything beneath them as they sink to the bottom of the ponds (HATCH, 2019a). Electro-fishing involves placing electrodes on a drag net or using hand-held electrical gear; the electrical discharge makes shrimp jump out of the water and land in the net (Cook & Rabanal, 1978a; Wickins & Lee, 2002, p. 158). Recently, more farmers have used mechanical harvesting, using various types of machines to pump shrimp out of the water (Ohs et al., 2006).
Once shrimp are out of the pond, they should be slaughtered. The study of slaughter methods for decapods has largely neglected small species like shrimp, instead focusing on larger ones, such as lobsters (Conte et al., 2021). Moreover, the slaughter of decapod crustaceans is mostly unregulated (Albalat et al., 2022; Conte et al., 2021), and there appear to be no standard practices in this domain. The FAO factsheets for P. vannamei, P. monodon, and M. rosenbergii suggest that all three species are most commonly slaughtered using an ice slurry (FAO, 2009a; 2009b; 2023c).
Farm characteristics
Ongrowing facilities are typically characterized as extensive, semi-intensive, intensive, or super-intensive, depending on their yield potential per unit area per time period. The FAO notes that these categories do not describe the actual diversity in operating practices implemented in different locations (Barg et al., 1999). That said, there are clusters of practices and conditions that roughly align with how intensively managed a farm is, which we list in Table 1 below. Note that any numerical estimate is relative to time and place, so our numerical ranges reflect our best understanding of contemporary differences in countries with the greatest amount of shrimp production.
Extensive and semi-intensive farms are located in tidal areas from which they source water. Extensive farmers rely on daily tidal cycles for water exchange, so there is little they can do when pathogens or low-quality water incidentally enters the pond. Extensive farms most closely match a shrimp’s natural habitat. For example, the earthen bottom of extensive ponds should allow shrimp to burrow if it is not too compacted (farmers compact soils to avoid erosion; Boyd et al., 2012)—the substrate on the bottom of the pond is usually the natural pond soil. In the wild, shrimp are subject to high predation pressure (Salini et al., 1990). During the day, some species dig into the soft sand bottom to hide from predators, with only their eyes protruding (Kenyon et al., 1995; Mariappan & Balasundaram, 2003; Wickins & Lee, 2002, p. 158). White shrimp (which includes P. vannamei) show less burrowing behavior than other groups, particularly brown shrimp (including P. monodon), who burrow daily (FAO, 2023c; Farfante, 1969; Pontes et al., 2006).
Semi-intensive farmers more closely monitor water quality and have greater control over it. They use pumps to bring water into the pond, so they can also pump it out when necessary. Semi-intensive farmers often store water in sediment ponds to reduce turbidity and break down organic matter (Lin & Drazba, 2006, pp. 79–82). They also occasionally use mechanical aerators to maintain dissolved oxygen levels.
Intensive and super-intensive farms aerate water more frequently and source it externally to ensure it is biosecure (FAO, 2009a). They also exchange it far less. Instead, Recirculating Aquaculture Systems (Shinji et al., 2019; Thakur & Lin, 2003) circulate water out of the ongrowing pond, through different treatment ponds (Box 3), and back into the pond. Both types of farms line their ponds to prevent diseases and erosion by aerator-generated water currents (Benchmark Insights, 2019, p. 54; Boyd et al., 2017), but liners likely also prevent shrimp from performing their normal burrowing behaviors. Both may also use biofloc systems, where bacteria are introduced to convert organic matter that would otherwise become toxic into edible biomass. Finally, super-intensive farms use indoor greenhouses, which prevents weather from influencing water quality.
Table 1. Characteristics of extensive, semi-intensive, intensive, and super-intensive penaeid shrimp farms. Based on Oddsson (2020), HATCH (2019b), and Boyd & Jescovitch (2020, pp. 243–244).
* Currently, only P. vannamei are reared in super-intensive facilities
‡ Specific pathogen-free (SPF) shrimp are from populations raised in biosecure facilities who have tested negative for certain pathogens for two consecutive years (Alday-Sanz et al., 2020).
Box 3: Pond and water treatment on shrimp farmsGenerally, water treatment is characteristic of intensive and super-intensive farms, but extensive and semi-intensive farms may use some treatments, such as antibiotics (Lyle-Fritch et al., 2006, p. 143). Recirculating aquaculture systems will use a variety of treatments so that used water can be reused in the same production cycle. There are a variety of treatments farmers use, below is a non-exhaustive list. Numbers in parentheses indicate whether the treatment is used for (1) water quality improvement or (2) disease prevention or treatment. |
||
|
Antibiotics (2): Farmers use antibiotics to treat shrimp
bacterial diseases. Prophylactic antibiotic use appears uncommon in ongrowing
systems but occurs more in hatcheries (Hinchliffe et al., 2018; Rico et al., 2013).
Biofloc (1): Biofloc systems add a microorganism community (e.g., phytoplankton, bacteria) to the pond, fulfilling various roles, including ammonia removal and acting as additional food for shrimp (El-Sayed, 2021) Disinfectants (2): Disinfectants, including chlorine, hydrogen peroxide, and some liming products (see below) are used to kill pathogens in the pond (Gräslund et al., 2003, pp. 83–83). Emergency oxygenation (1): Hydrogen peroxide is sometimes used when pond oxygen levels drop dangerously low and an emergency source of oxygen is required (Furtado et al., 2014). Fertilizers (1): Used to improve the growth of plankton in the pond, fertilizers help improve water quality and produce a food source for shrimp (Gräslund et al., 2003, p. 82). |
Filtration (1, 2): Biofilters and solids filters remove
unwanted bacteria, phytoplankton, and suspended solids from the water. Examples
of biofilters include oysters and tilapia (Jones et al., 2002;
Tran et al., 2014).
Liming (1): Liming products increase the alkalinity of the pond and water and may be used before ponds are filled with water and stocked with shrimp, or during the farming cycle. Probiotics (1, 2): Aqueous probiotics improve organic matter decomposition in the water, which improves water quality and prevents the proliferation of pathogenic bacteria (Alune, 2021a). Pesticides (2): Farmers apply pesticides to remove predators, parasites, and species who can act as disease vectors from the pond (see Diseases and Water pollutants sections). Siphoning (1): Siphoning removes sludge (made up of things like feces, dead phytoplankton, and leftover food), improving the conditions of the pond bottom (Alune, 2021b). |
|
Stocking density increases with farm intensity, another way in which more intensive farms are less like shrimp’s wild environments. In the wild, shrimp would not be expected to aggregate in groups while they are burrowing, as this would undermine their camouflage (Evans et al., 2007). However, species who do not burrow or are at least not currently hiding might aggregate in groups, as this could reduce the likelihood of predation at the individual level (Krause & Ruxton, 2002, Ch. 2). There are conflicting accounts of whether wild penaeids exhibit aggregating behavior. Bauer (2010) reports that “Penaeoidean and many caridean species are highly mobile and live in dense aggregations, resulting in frequent contacts among individuals,” while others report that such behavior is rare in most penaeids (Lucas et al., 1979). Rivera-Velázquez et al. (2008) found that wild P. vannamei juveniles were present in a coastal lagoon in Mexico at average densities of 0.302m-2 (see Medina-Reyer, 2001 for slightly higher estimates), though seasonality played a large role: during the rainy season, densities at one point reached 4.9m-2. This is consistent with shrimp moving towards favorable environments, with aggregation as a byproduct rather than an instinct that evolved to avoid predation (Evans et al., 2007). Extensive farms most closely match the densities of shrimp found in the wild. As the shrimp farming industry becomes more intensive, more shrimp will experience more environments that differ from their natural ones.
Few estimates exist concerning the proportion of global shrimp output produced by each type of farm. An expert from the industry interviewed for this report estimates that, in 2020, around 5% of the worldwide output may come from extensive farms, 30% from semi-intensive, 60% from intensive, and finally, 5% from super-intensive operations. However, from a more comprehensive analysis of available data, Boyd et al. (2022, p. 33) conclude that, in 2018, the proportions of shrimp production from each system type were as follows: 11.5% extensive, 16.5% semi-intensive, and 71.8% intensive. As the authors mention hyper-intensive (c.f. super-intensive) facilities elsewhere in the report, we assume they are suggesting that this type of farm accounted for less than 1% of shrimp production that year. Note that with the trend towards intensification, this may have changed by now.
Welfare threats
Diseases (domain 3: health)
Annually, viral infections are responsible for substantial losses in tropical shrimp aquaculture production, and disease is persistently identified by shrimp farmers as the biggest industry challenge (Anderson et al., 2016a; 2016b; 2017; 2018; 2019; HATCH, 2019c; Stentiford et al., 2012). The large burden of disease partly reflects the fact that disease is a downstream consequence of many of the other welfare issues we will discuss in subsequent sections.
Shrimp are also inherently vulnerable to disease because, like all crustaceans, they do not have an adaptive immune system like that of vertebrates, only an innate immune system (Arala-Chaves & Sequeira, 2000; Vazquez et al., 2009). Because shrimp generally cannot be immunized against diseases, the industry has only three preventative strategies left. First, they can source disease-free shrimp. The industry is increasingly dependent on domestically-bred, Specific-Pathogen Free (SPF) seed shrimp. SPF shrimp are those from populations raised in biosecure facilities, with biosecure feeds, that have tested negative for certain shrimp pathogens for two consecutive years (Alday-Sanz et al., 2020). SPF status does not indicate anything about tolerance to pathogens, instead only referring to an absence of disease (Eswaran, 2021). Some facilities claim to be breeding specific-pathogen resistant and tolerant shrimp,7 though an expert told us these are not yet widely available in the industry.
Second, farmers can use medicine to treat or prevent infection (see Box 3). Some use antibiotics, but this has resulted in the emergence of antibiotic-resistant strains (Vaiyapuri et al., 2021). Probiotics improve the gut microbiota of shrimp or pond water (Alune, 2021a), bolstering shrimp immunity to bacterial diseases (Knipe et al., 2020, pp. 325–327).
Third, farmers can maintain a disease-free environment. Wu et al. (2001) showed that when diseased carcasses were immediately removed from tanks, there was a lower mortality rate than when they were removed at regular intervals in a tank of the same density (see also Hamano et al., 2015). The World Organization for Animal Health (WOAH) notes in the Aquatic Animal Health Code (WOAH, 2022, 4.1.7) that pathogens may enter a shrimp farm via other animals, water, feed, and farm equipment. When multiple farms use a common water source, disease incidence at one farm can easily spread to another (Benchmark Insights, 2019, p. 53; Tendencia et al., 2011). Similarly, an expert interviewed for this report claimed that insects easily act as vectors of pathogen agents when farms are close to one another. Also, some water contaminants may reduce the immune responses of shrimp (see Water pollutants section). Where biosecurity measures fail and it is not possible or cost-efficient to treat the stock, farmers may use depopulation methods and then begin the farming cycle again from scratch.
Significant infectious diseases
The WOAH lists significant aquatic animal diseases (WOAH, 2022, Section 1.2) that:
- Can be detected and diagnosed
- Have spread internationally
- Severely impact industry productivity, wild animal health, or human health (in the case of listed shrimp diseases, all appear to have impacted industry growth rather than wild animal or human health)8
Of the 11 aquatic crustacean diseases listed by WOAH, ten affect farmed shrimp. These are listed in Table 2 below, along with environmental risk factors, effects on shrimp, transmission routes, and mortality rates.
Table 2. Main diseases in farmed shrimp. Based on World Organisation for Animal Health Aquatic Animal Health Code Sections 2.2.1., 2.2.3., 2.2.4., 2.2.5., 2.2.6., 2.2.7., 2.2.8., 2.2.9., and disease cards for Enterocytozoon hepatopenaei and Decapod iridescent Virus 1.
*We list only those susceptible out of the most farmed species (P. vannamei, P. monodon, and M. rosenbergii). Other shrimp species may be susceptible. Where a species is listed as ‘maybe’ being susceptible, the WOAH has listed these as having “incomplete evidence for susceptibility.”
Water quality (domains 2, 3: environment, health)
Critical physiological tasks like respiration and enzymatic reactions rely on particular concentrations of chemicals in the water surrounding shrimp. Although shrimp have adaptations for maintaining homeostasis, energy used to preserve optimal functioning cannot be allocated towards other physiological tasks, such as fighting infections (Clayton et al., 2022, p. 296). As a result, suboptimal water quality may reduce growth and increase susceptibility to disease.
Here, we focus on five parameters that are fundamental to shrimp health: dissolved oxygen (DO), temperature, salinity, pH, and ammonia (Boyd & Fast, 1992, pp. 497–500; Kungvankij et al., 1986b; Le Moullac & Haffner, 2000). Separate from these water quality parameters, we also discuss water pollutants, including pesticides and heavy metals. We present standards for what the water quality parameter concentrations should be in Table 3. Note that acceptable levels for larvae and young postlarvae are usually more restrictive since these animals are often more sensitive and have different environmental requirements than juveniles and adults. Optimal ranges are typically less restrictive during intermolt periods when shrimp have a hard exoskeleton.
The recommended standards evaluate each dimension of water quality in isolation from one another. In reality, the optimal level of some parameters depends on the values of others. For example, high temperature results in lower DO levels because warmer water holds less oxygen, while higher salinity reduces DO levels because salt makes oxygen less soluble (Boyd & Fast, 1992, p. 501; Lazur, 2007). The way shrimp react to certain parameters can also affect other parameters. For instance, shrimp consume more oxygen at higher temperatures, increasing the rate at which DO depletes (Hargreaves & Boyd (2022, p. 208). Water quality standards ignore these complexities to make water quality monitoring simpler.
We extracted data from surveys to estimate the extent to which farms maintain water quality within the recommended ranges. For each study, we estimated the average percentage of the time that water quality is "optimal" (i.e., no negative welfare effects) and "suboptimal" (i.e., negative welfare effects that are either sublethal only or cause lethality at rates that are typical in natural conditions), from which we can also infer how often it is unacceptably low. The complete analysis, including a discussion of the limitations of available data, is available in our supplementary document, Water Quality in Shrimp Ongrowing Ponds; the results are summarized in figures in the subsections below.
Table 3. Recommended levels of water quality parameters for penaeids and M. rosenbergii in ongrowing ponds. Recommendations for penaeids are from Pedrazzani et al. (2023, Table 11) unless otherwise stated, and those for M. rosenbergii are from New (2002, p. 19).
Dissolved oxygen (DO)
Respiration requires oxygen, which shrimp obtain from the water through their gills. Beyond a certain concentration of DO, there is no additional benefit of greater amounts of it. However, the point at which DO consumption is independent of DO concentration varies according to how active shrimp are—after feeding, during the post-molt stage, and while swimming, respiration rates are much higher (Hargreaves & Boyd, 2022, pp. 211–214).
In extensive and semi-intensive ponds, most DO is a by-product of photosynthesis by plants and phytoplankton in the pond. Because phytoplankton also respire, they consume more DO than they produce when there is little photosynthesis. Accordingly, shrimp "are often exposed to hypoxic conditions" due to a lack of sunlight at night (Hargreaves & Boyd, 2022, p. 218).
Less obviously, phytoplankton near the surface can block sunlight from reaching plants and algae beneath the surface. Farmers strive for an intermediate amount of phytoplankton so that there is enough photosynthesis to meet the demand for DO but not so much that they shade each other.
In intensive systems, there is greater oxygen demand due to higher stocking densities and the introduction of biofloc. Moreover, bacteria begin displacing phytoplankton over the course of production, so farmers need mechanical aerators to increase DO levels. The paddlewheel aerators most frequently used do a better job at increasing dissolved oxygen near the surface than towards the bottom, where shrimp often prefer to dwell. Some farmers also use aerators designed to reduce stratification, but they can result in strong water currents around the periphery and near-stagnant waters in the middle. The periphery is well-mixed but is not necessarily desirable because shrimp must swim against the current to stay in place. Conversely, the current in the middle is less rapid but more anoxic (Hargreaves & Boyd, 2022 pp.251–254).
Low DO levels reduce shrimp growth and molting frequency (Allan & Maguire, 1991; Boyd, 1989, p. 25; Kungvankij et al., 1986b; Nonwachai et al., 2011). In ponds with chronically low DO concentrations, shrimp will eat less and convert food to flesh less efficiently (Boyd, 1989, p. 25). Low DO may also reduce swimming ability (Duan et al., 2022). Zhang et al. (2006) observed that as DO levels reduced, P. vannamei showed random short bursts of swimming and, as conditions became severe, exhibited clear surface-seeking behavior before being still at levels below 1 mg L-1.
As DO decreases, shrimp will experience serious physiological effects leading to suffocation (Boyd & Zimmermann, 2010, p. 240) and, subsequently, to death (at 0.9 mg L-1 for P. monodon; 1.2 mg L-1 for other penaeids) (Allan & Maguire, 1991; Boyd, 1989, p. 25; Cook & Rabanal, 1978b; Madenjian et al., 1987). McGraw et al. (2001) report that at DO levels below 4 mg L-1, mortality rates of postlarvae were between 45% to 58%, after five months. Low oxygen levels also hamper shrimp immune systems, making them more susceptible to disease (Boyd, 1989, p. 25; Kungvankij et al., 1986b; Le Moullac & Haffner, 2000; Nonwachai et al., 2011). On the contrary, high DO levels (above 4–5 mg L–1) improve shrimp survival and growth (McGraw et al., 2001; Nonwachai et al., 2011).
Our summary of water quality studies suggests that DO is rarely at a dangerously low level (Figure 5). On the other hand, sublethal effects of low DO are not uncommon. The earliest study (published in 2006, but based on 2001 data) is the most concerning. It could be that DO levels have improved over time, or that methodological differences between the studies explain the discrepancy. Additionally, many farmers do not measure DO at all (Boyd et al., 2018, p. 567), so suboptimal DO levels that cause sublethal welfare effects may be underreported.
Figure 5: Estimates of the average percentage of the time that shrimp farms maintain optimal or suboptimal dissolved oxygen levels. Optimal and suboptimal are defined as Pedrazzani et al.’s (2023) Score 1 (ideal limits) and Score 2 (sublethal effects), respectively. For more detail see our quantitative analyses of water quality in shrimp ongrowing ponds.
Temperature
Shrimp are poikilothermic or ‘cold-blooded’ animals, regulating their temperature via their behavior. As with other poikilothermic organisms, higher temperatures increase shrimp metabolism (Boyd & Zimmerman., 2010, p. 240; Kungvankij et al., 1986b). When the temperature is too low, shrimp may not have the energy to carry out routine activities like feeding (Ren et al., 2021). P. vannamei undergo physiological changes to maintain sufficient energy absorption during cooling periods (Wang et al., 2019). However, when kept at 13˚C for 24 hours, apoptosis (programmed cell death) and hepatopancreas damage occurs.
P. vannamei endure oxidative stress when at 33°C for 24 hours or more, which diminishes their immune response and damages their intestinal barrier (Duan et al., 2018a). Lower temperatures may also compromise the immune response of P. vannamei, making them more susceptible to infection (Pan et al., 2008; Ren et al., 2021; Wang et al., 2020). However, temperature deviations are not always negative. Many pathogens may be less virulent and transmissible at cooler temperatures. Conversely, high temperatures can improve resilience to white spot syndrome among infected shrimp (You et al., 2010). Fluctuations in temperature are probably more harmful when they are sudden (Dayalan et al., 2022; Jia et al., 2014).
|
Box 4: Thermal preference studiesResearchers test the thermal preferences of animals by allowing them to move within an environment that varies in temperature across space, while other aspects of water quality are held constant (e.g., Diaz et al., 2002). These studies usually also forcibly expose animals to non-preferred temperatures to identify the "critical thermal maximum", and minimum. These thresholds are the point at which animals lose the ability to move in a desired direction, show a loss of the "righting reflex," or the ability to maintain balance (Hoang et al., 2002, p. 23; Kumlu et al., 2010, p. 303) This study designs raise two important points about the relationship between welfare and observable behavior. First, preferences can only provide an ordinal ranking of different conditions, not an absolute measure of welfare. While the preferred temperature presumably creates positive welfare and the temperatures beyond the critical point presumably result in negative welfare, it is indeterminate whether the range of temperatures in between creates positive or negative welfare. As Dawkins (1977, pp. 1041–1042) points out, when animals have multiple options, they will choose the best option even if the other options are also rewarding. Non-behavioral evidence would be required to show that a non-preferred but sublethal temperature is reducing welfare. Second, the metrics that farmers optimize for do not necessarily maximize welfare. For example, Chen & Chen, 1999 note that the temperature preference of postlarval P. monodon shrimp (20–25.6ºC, mean of 23.2˚C) is below their optimal growth temperature for the species. The authors suggest that individuals may choose a lower temperature to conserve energy, given uncertainty about food availability and the potential negative effect of high temperatures on survival. González et al. (2010) argue that optimization for growth depends on life stage: P. vannamei juveniles do select temperatures based on growth, whereas adult preferences are driven by investment in reproduction. Overall, growth typically is evidence of good health, it may be at the expense of the quality of experience. The present report uses growth as one proxy among many for good welfare, mostly because it is almost always measured in studies of aquaculture. Studies that are designed from a welfare perspective should measure behavioral preferences, even though they are imperfect measures on their own. |
|---|
The temperature in ponds can fluctuate daily and with the seasons (Lin & Drazba, 2006, p. 79). Transient effects like rainfall can also cause water temperature to drop in outdoor ponds (Buike, 2018). Pond depth also affects the water temperature—greater depth can protect shrimp from high temperatures (Kutty, 1987). Shrimp have thermal preferences (see Box 4) and neurons to detect cold (Tani & Kuramoto, 1998), suggesting that they would take advantage of lower depths if they felt too hot.
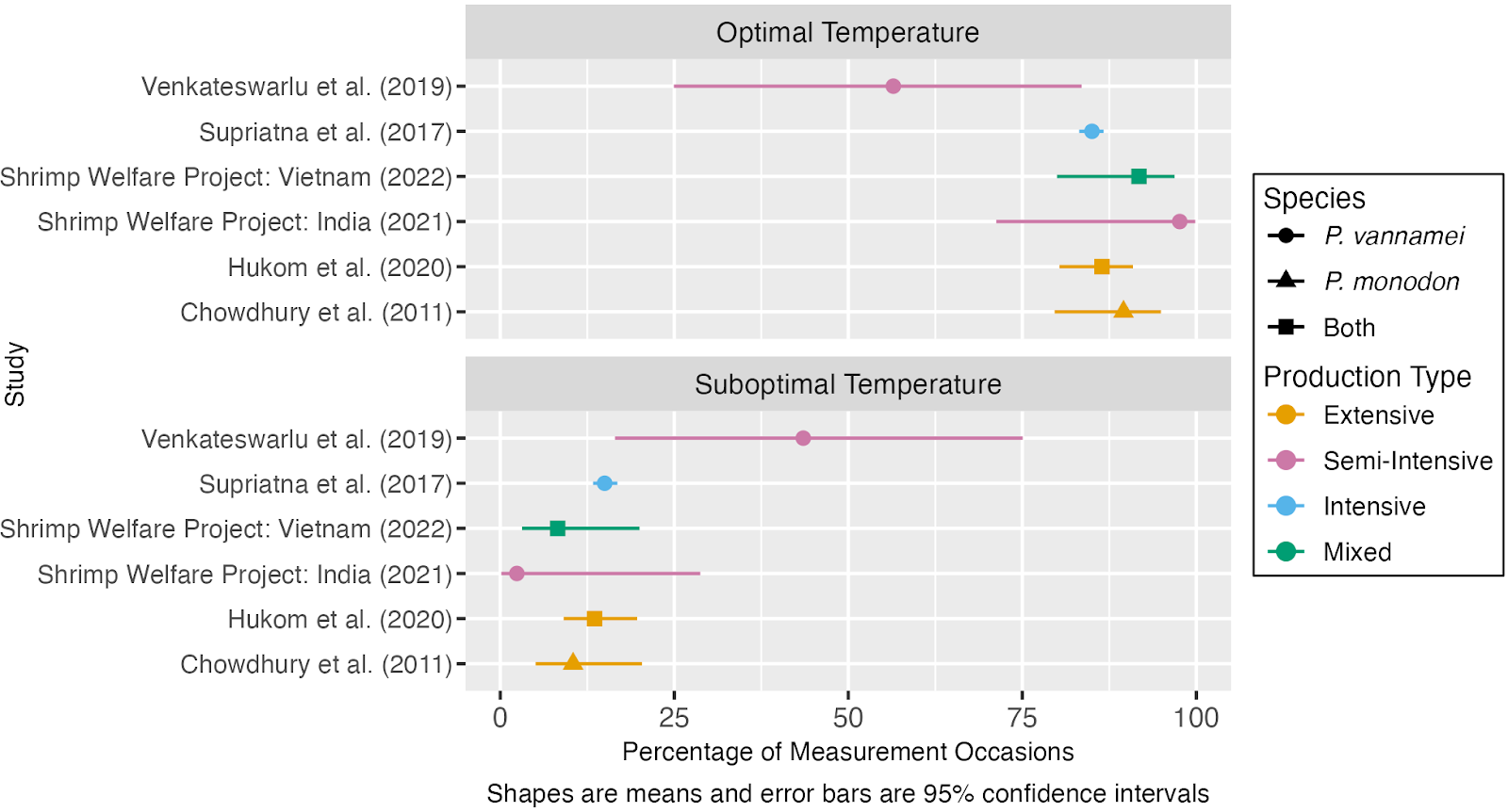
Our quantitative analysis of reports of water quality in shrimp ongrowing ponds suggests that temperature is within the optimal range around 80% of the time on average and suboptimal around 12% of the time. The outlier is Venkateswarlu et al. (2019), where temperatures were roughly split between optimal and sub-optimal, and were practically never unacceptable. Examination of this dataset suggests that low temperatures are probably a more common issue than high temperatures. This is surprising insofar as farmers avoid stocking shrimp during cold spells or the cold season (Lin & Drazba, 2006, p. 79). On the other hand, the datasets with an appreciable frequency of suboptimal temperatures more often show high temperatures.
Figure 6: Estimates of the average percentage of the time that shrimp farms maintain optimal or suboptimal temperatures. For more detail see our quantitative analyses of water quality in shrimp ongrowing ponds.
Salinity
For shrimp, homeostasis requires matching the salinity level of the surrounding water rather than maintaining a certain internal salinity level. When external salinity is lower, water will be drawn into the shrimp's cells. If the inflow continues unabated, cells will swell and eventually burst. Conversely, if the external salinity is higher, water will be drawn out of cells and into the external environment. If too much water leaves, cells will shrink and lose their structure. Shrimp have mechanisms for adjusting their internal salinity, but these adjustments take time, so rapid changes in salinity can cause “osmotic shock,” potentially leading to death. The ideal external salinity for shrimp is their isosmotic point—the internal salinity they do not need to expend resources to maintain (Roy et al., 2010). The isosmotic point for P. vannamei is about 25 ppt (Clayton et al., 2022 p. 292).
Extensive and semi-intensive farms typically take their water from estuaries, the salinity of which changes with season. Within a production cycle, salinity declines during the rainy season and increases in the dry season—farms that do not regularly exchange and treat their water will be vulnerable to these fluctuations (Boyd & Fast, 1992, p. 501). In climates with dry summers, a high evaporation rate can gradually increase the usual salinity levels (Kungvankij et al., 1986b).
Intensive and super-intensive farms are often indoors, shielding them from weather-mediated salinity changes. They also often use low-salinity water, as a cost-saving measure (Boopathy et al., 2007; Roy et al., 2010). Shrimp in these systems are likely acclimatized to low-salinity conditions prior to being put in ponds, which would negate some of the negative impacts (Roy et al., 2010; Suantika et al., 2018).
According to Boyd & Tucker (1998, p. 94), if shrimp are reared outside the recommended ranges for their species and development stage, “performance is diminished and survival may be poor”. Penaeids are marine animals, so we expect greater sensitivity to low salinities. Indeed, growth and survival tend to decrease with decreasing salinity (Chen et al., 2016; Gao et al., 2016; Laramore et al., 2001). That said, salinities above 60 ppt can be lethal even for penaeids (Boyd & Fast, 1992, p.497; Pedrazzani et al., 2023, Table 11). Nevertheless, we expect overly high salinities to be rare on farms: the water used by extensive and semi-intensive farms is usually brackish or coastal, and water sourced by inland farms (intensive and super-intensive) is generally of low salinity (Boopathy et al., 2007; Roy et al., 2010). Moreover, deviations in either direction are unlikely to have catastrophic consequences for penaeid shrimp, so long as the change is not too sudden. Part of the reason P. vannamei are so popular to raise on farms is that they can tolerate a very wide range of salinity levels (Boyd & Tucker, 1998, pp. 99–100; Chen et al., 2016; McGraw et al., 2002).
Our quantitative analysis of water quality in shrimp ongrowing ponds suggests that optimal salinity levels are less common than optimal levels of other water quality parameters. Instead, salinity is more often at suboptimal levels. It seems possible that farmers make less of an effort to maintain ideal salinity levels, possibly because they know penaeids can tolerate wide salinity ranges. Therefore, even though nonoptimal salinity may be more prevalent than other water quality concerns, the effect on shrimp welfare may be less substantial, relative to other water quality parameters. Note, however, that the data cannot speak to the prevalence of unacceptably fast changes in salinity levels.
Figure 7: Estimates of the average percentage of the time that shrimp farms maintain optimal or suboptimal salinity levels. For more detail see our quantitative analyses of water quality in shrimp ongrowing ponds.
pH
The pH scale measures the water acidity and is usually represented as ranging from 0 to 14. Lower pH values correspond to more acidic conditions, while higher pH values correspond to more basic or alkaline solutions, and a pH of 7 represents neutral water. Because pH levels affect the shape and structure of enzymes, deviations from optimal pH can reduce the functionality of proteins (Clayton et al., 2022, p. 296). A pH between 6.5 and 8.5 is suitable for P. vannamei shrimp (Pedrazzani et al., 2023, Table 11).
Pond soils may be acidic because they contain high concentrations of organic matter or acidic clay (Boyd & Fast, 1992, p. 506–507). Other factors like rain, which is normally acidic, can also affect pH levels in outdoor ponds (Boyd & Tucker, 1998, p. 224)—when evaporation exceeds rainfall, the water will become more alkaline.
Respiration reduces pH levels because carbon dioxide combines with water to form carbonic acid. Consequently, pH decreases at night when algal photosynthesis decreases and shrimp activity increases (Boyd, 2017a; Lazur, 2007). Similarly, higher stocking densities reduce pH because there are more shrimp and more microbes added in to detoxify ammonia. To make ponds more alkaline, farmers carry out liming during pond preparation before stocking and to the water during production cycles (Boyd, 2017b; Kungvankij et al., 1986c).
As alluded to in Box 2, there is debate over whether shrimp have aversive reactions to acids. Even if shrimp do not have nociception for pH, extreme levels could still impact welfare by reducing health. Extreme pH levels can reduce feeding, growth, and immune function (Boyd, 2017b; Kubitza, 2017; Kungvankij et al., 1986b; Yu et al., 2020). Mortality and oxidative stress also seem to increase at extreme pH values of 4 and below, and 9.5 and above (Furtado et al., 2015). Chronic deviations from recommended pH ranges in either direction reduce survival in shrimp, while reductions in growth appear more pronounced for high pH (Yu et al., 2020). However, gradual exposure to low pH may provide some protection against bacterial infection for P. vannamei (Han et al., 2018).
Our summary of water quality studies suggests suboptimal pH levels about 15% of the time and otherwise optimal levels (Figure 8). The exception again is Venkateswarlu et al. (2019), where pH is optimal only around 60% of the time, and is unacceptable around 15% of the time. Examination of this dataset suggests that overly high pH may be slightly more common than pH levels that are too low.
Figure 8: Estimates of the average percentage of the time that shrimp farms maintain optimal or suboptimal pH levels. For more detail see our quantitative analyses of water quality in shrimp ongrowing ponds
Ammonia
Shrimp excrete ammonia as a by-product from metabolizing protein in their food. The most important cause of ammonia accumulation in aquaculture is decomposing organic matter (decaying microalgae, shrimp feces, organic fertilizers, and unconsumed feed). Ammonia exists in water in two forms: as un-ionized ammonia (NH3) and as the ammonium ion (NH4+). Toxicity is related principally to un-ionized ammonia because of its greater permeability into the hemolymph, the invertebrate analog of vertebrate blood (Boyd & Tucker, 1998, p. 48). pH determines the percentage of ammonia that occurs un-ionized (Kubitza, 2017; Kungvankij et al., 1986b). Similarly, a given concentration of un-ionized ammonia is more toxic when DO (Allan et al., 1990) or salinity concentrations (Lin & Chen, 2001) are low.
The accumulation of un-ionized ammonia has several negative consequences, such as lower levels of feeding (Frias-Espericueta et al., 2000). The main issue, though, is that un-ionized ammonia increases the likelihood of infection via several different paths. Ammonia damages shrimp gills, increasing susceptibility to viruses that target the gills (e.g., WSSV; Clayton et al., 2022, p. 306). Ammonia also damages the intestine barrier (Duan et al., 2018b), which normally prevents pathogenic invasion (Zhao et al., 2020). High ammonia concentrations also slow down hemolymph coagulation, allowing microbes to enter open wounds (Chang et al., 2015). Finally, high ammonia concentrations cause hemocyte apoptosis, directly reducing immune capacity (Liu et al., 2020). At high enough levels in the hemolymph, ammonia directly causes mortality (Boyd & Fast, 1992, p. 498; Boyd & Tucker, 1998, p. 135; Kubitza, 2017). Signs of impending death include hyperactivity, convulsions, and then lethargy and coma (Boyd & Tucker, 1998, pp. 134–136).
Although several datasets considered in our quantitative analysis of water quality studies measured total ammonia levels, very few measured un-ionized ammonia levels. Nevertheless, our analysis suggests that sublethal and lethal ammonia levels could be worryingly common (Figure 9).
Figure 9: Estimates of the average percentage of the time that shrimp farms maintain optimal or suboptimal un-ionized ammonia levels. For more detail see our quantitative analyses of water quality in shrimp ongrowing ponds.
Water pollutants
Within the farming pond, shrimp can also be exposed to chemicals that are either applied purposely by farmers or arise through other means, like agricultural runoff or water source pollution. We cover pesticides and heavy metals, as these were the issues we found the most information on.
Shrimp farms located in or near industrial areas or agricultural zones are most likely to be affected by pesticide runoff, either directly into outdoor farming ponds or through contaminating the water source used by a farm. Some farmers may also purposely apply these chemicals to prevent disease or invasion by other species into the pond. Of 164 shrimp farms surveyed by Rico et al. (2013) in Bangladesh, China, Thailand, and Vietnam, 12 applied parasiticides (which included some insecticides; see Table 2 in Rico et al., 2013). As shrimp are phylogenetically close to insects, with both in the phylum Arthropoda, they are vulnerable to damage from insecticides. The authors also reported that parasiticides are commonly sold in shrimp farm supply shops in Bangladesh, Vietnam, China, and, to a lesser extent, Thailand. Heal et al. (2021) report that 63% of farmers surveyed in 2016 in Bangladesh used pesticides (see Table 3 in Heal et al., 2021). Insecticides have also been found in shrimp farms in Mexico and may have contributed to reduced productivity in these areas (Burgos-Hernández et al., 2006). In Ecuador, 28 of 101 surveyed farms applied piscicides to their ponds (Boyd et al., 2021). As there is scant literature on the concentration at which pesticides are toxic, it is unclear if farmers are applying enough of these products to impact shrimp.
It has long been recognized that agricultural runoff of pesticides can be harmful to aquatic life (e.g., see EFSA, 2013). Several types of pesticides can affect a shrimp's health status. In P. vannamei, fungicides can have adverse effects on the immune system (Muñoz et al., 2000) and those exposed to sublethal concentrations of pesticides exhibit abnormal swimming, hyperactivity, and spasms (García-de la Parra et al., 2006). For P. monodon, Hook et al. (2018) found that pesticides significantly affect the survival of postlarvae and that some types inhibited feeding behavior when used at non-lethal levels. Additionally, the authors found that water sources used by shrimp farms in Australia contained levels of these pesticides that would impede growth or survival.
Agricultural runoff and purposefully applied chemicals, like fertilizers or algaecides, can also introduce heavy metals into shrimp ponds (Bautista-Covarrubias et al., 2022; Qian et al., 2020). Heavy metals may also be present in shrimp feed (Islam et al., 2017; Lyle-Fritch et al., 2006). Some heavy metals, like zinc and copper, are essential for normal shrimp growth, but even these can cause adverse effects and mortality in high doses (Zhou et al., 2022, pp. 377–378). In general, heavy metals can cause physiological alterations in shrimp, such as disruption of osmoregulation (Frías-Espericueta et al., 2008) and gill damage, which can cause death by asphyxia (Frías-Espericueta et al., 2008; Li et al., 2007). Heavy metal stress can make shrimp more susceptible to diseases (Bautista-Covarrubias et al., 2022). Chronic exposure to copper reduces growth and immunity in P. vannamei (Qian et al., 2020). Additionally, the presence of multiple heavy metals at levels considered to be safe when present individually can have similar effects to lethal doses of any one heavy metal (Frías-Espericueta et al., 2008).
We were unable to find information about how much recirculating aquaculture systems and intensive farms are exposed to this issue. However, it is reasonable to assume that these water pollutants are less common in these systems because water is typically treated before use and many intensive farms are indoors, reducing the risk of insects accessing the farming area and therefore the need for insecticides.
Stocking density (domains 2, 3, 4: environment, health, behavior)
Farmers who adopt intensive production methods can employ higher stocking densities because they provide more aeration and food than is available in extensive ponds (see Table 4). High stocking densities worsen water quality. Fortunately, intensive farms have a greater capacity to manage water quality degradation. However, high densities reduce growth even when water quality parameters are carefully controlled (da Silveira et al., 2020), suggesting that crowding per se threatens welfare. In particular, crowding increases vulnerability to aggression and disease, and potentially frustrates preferences. Because these latter issues are inherent to crowding, they can only be addressed by reducing stocking densities.
Table 4. Estimated area available per P. vannamei postlarval (PL) shrimp under different ongrowing systems. Based on FAO (2009a).
*FAO (2009a) reports super-intensive stocking densities for juveniles only; here, we assume that the density is similar for postlarvae. Upper-bound limits may have increased since FAO (2009a) was written (e.g., da Silveira et al., 2020 and this video of a super-intensive farm, which uses a stocking density of 500 per m2).
Water quality degradation
Higher stocking densities mean more uneaten feed and excrement, which raises ammonia levels. da Costa et al. (2016) found that elevated mortality rates at densities of 100m-2 could be attributed to ammonia toxicity (see also Nga et al., 2005). Because intensive farms exchange their water less, they do not have a natural mechanism for removing ammonia from tanks. One solution is to implement a biofloc system. However, microbes complicate water quality management because they need oxygen too. Krummenauer et al. (2011) reported that dissolved oxygen showed an abrupt decrease after about 90 days of rearing P. vannamei with biofloc at intensive and super-intensive levels. This change comes after microbes begin to predominate over algae at around 64 to 70 days (Hargreaves & Boyd, 2022, p. 228).
Meanwhile, an increased reliance on mechanical aeration poses risks of its own. Aerators tend to push sludge to the center of the tank (Delgado et al., 2003), which consumes oxygen that would otherwise be used for shrimp respiration. The anoxic environment in turn creates anaerobic byproducts including ammonia. Intensive farms can, however, install a drain or siphon to remove ammonia (Kubitza, 2017).
Our survey of farm water quality suggests that intensive farms may generally succeed in managing dissolved oxygen levels (see Figure 5). However, there was very little data on intensive (especially super-intensive) farms near the end of the crop cycle. Also, any system reliant on mechanical aeration risks a disaster if the aerators suddenly stop working, due to a mechanical problem or power failure.
Increased vulnerability
Even among species who do aggregate, they typically will not crowd as closely together as is physically possible because proximity comes with its own costs (Krause & Ruxton, 2002, Ch. 3). The most readily visible issue is aggression, which can occur due to territoriality or hunger. For P. monodon, cannibalism increases with density (Abdussamad & Thampy, 1994; Jiang et al., 2021), possibly because it is easier to detect an easy target. Decapod crustaceans are particularly vulnerable to cannibalism when they do not have a hard exoskeleton, which routinely occurs after molting but can also result from disease or poor water quality (Romano & Zeng, 2017).
The greater aggressiveness of M. rosenbergii and P. monodon explains why farmers raise them at lower densities (Valenti et al., 2009a, p. 168; Wickins & Lee, 2002, p. 15). It is likely uncommon for P. vannamei shrimp to consume conspecifics who are still alive (Araneda et al., 2008; da Costa et al., 2016; Schroeder et al., 2010), but they do form dominance hierarchies (Bardera et al., 2021). In crowded conditions, subordinate individuals likely have less energy to allocate to growth, since they have less access to food and space to rest (Araneda et al., 2008, p. 16). This could in turn result in physical heterogeneities, further intensifying hierarchies (Bardera et al., 2021; Lord et al., 2021, p. 3). The conflicts that establish these hierarchies are presumably negative on the whole, at least for losing individuals.
Frustrated preferences
If proximity indeed increases vulnerability, then shrimp should have evolved to prefer a certain amount of space. Given that wild shrimp aggregate at levels that are at most consistent with densities on extensive farms, densities in more intensive farms may frustrate their preference. In support of the possibility, da Costa et al. (2016) report that retreat behaviors (i.e., moving away from other shrimp) are less common with increasing density. The authors suggest that shrimp value keeping a certain distance from each other, but cannot do so without simply getting closer to other individuals (pp. 919–920).
Personal space may also be an incidental requirement for other behavioral needs. For benthic species, rest and burrowing both require access to the bottom of the tank or pond. We examined whether shrimp movement would be restricted on a two-dimensional plane in different ongrowing systems, on the optimistic assumption that environmental parameters are suitable throughout the bottom of the tank. We use P. vannamei for these calculations, as they are stocked at the highest density (Boyd et al., 2017, Boyd et al., 2018). A mature P. vannamei postlarvae (after 22 days in the postlarval stage) is approximately 1.5 cm long (Teixeira & Guerrelhas, 2014). Adults can get as long as 23 cm (FAO, 2009a; Panutrakel & Senanen, 2021). If the width-to-length ratio is about 0.16, then postlarvae occupy an area of 0.36 cm², while adults occupy an area of around 84.64 cm². Postlarvae should have ample room to turn around even in super-intensive systems, whereas adults would run out of room in more densely packed intensive systems. In intensive systems, the initial number of shrimp per pond may be reduced later as the animals grow—for example, when shrimp reach 5g, the density may be lowered to 140–150/m2 (Wickins & Lee, 2002, p. 155). However, at 140 adults per m2, there is only about 71 cm² per adult, which would prevent shrimp from all simultaneously accessing the bottom. That said, we are unsure what percentage of shrimp want to rest at any given time.
Environmental enrichment (domains 2, 4: environment, behavior)
Not only are farm ponds often lined with plastic or concrete, but they also often lack shelters.9 One might infer that such a barren environment constitutes behavioral deprivation, but it is unclear what kind of environments shrimp prefer. However, they might not be able to ‘switch off’ their tendency to be vigilant to predators, even if they live on a farm where predation is not possible. A lack of concealing structures or substrate to burrow inside of may make it harder for shrimp to feel protected from such threats. Hiding might also feel rewarding in its own right.
Bratvold and Browdy (2001) found that adding only sand to ponds holding P. vannamei had no effect on growth or survival compared to no-sand ponds, but having sand and artificial, vertical substrates (AquaMats) increased growth rates and survival significantly. The study did not include a no-sand with artificial substrate treatment, so it is unclear if this effect is due to both features being present or not. However, looking at other evidence, the addition of artificial substrates into tanks does appear to improve both the growth and survival of juvenile shrimp (P. monodon with bamboo segments: Anand et al., 2019; P. vannamei with mesh screens: Schveitzer et al., 2013; Sofyani & Sambhu, 2020)—though note that one study found no significant differences (Fleckenstein et al., 2020). Survival and growth of postlarvae in the nursery phase also appear improved by adding substrates (Tierney et al., 2020; Zhang et al., 2010). When offered substrates, shrimp choose to spend more time on the substrates, and the number making such a choice increases over time (Zhang et al., 201010). Rearing shrimp with substrates also increases shrimp survival, growth, biomass, and feed conversion ratios, possibly because the increased surface area allows them to rest more or because shrimp can remain further apart, reducing crowding stress (Kring et al., 2023; Schveitzer et al., 2013; Tierney et al., 2020; Zhang et al., 2010). Mortality also likely decreases because substrate provision decreases cannibalism regardless of stocking density (Abdussamad & Thampy, 1994).
Shrimp farm environments may also not match shrimp color preferences. To the best of our knowledge, ponds that are lined are mostly lined with black or blue plastic11 or concrete. While evidence is sparse, P. vannamei shrimp appear to have a preference for red and yellow substrate, as opposed to blue or green, and feeding and growth rates were also improved in their preferred conditions (Luchiari et al., 2012). Kawamura et al. (2017) suggest that these findings cannot be explained by phototaxis (attraction to light or brightness) alone.
M. rosenbergii show a preference for black shelters, or when females are reared without males, red or orange shelters (dos Santos et al., 2015). Kawamura et al. (2017) found that M. rosenbergii postlarvae also prefer black shelters—though this study did not include red, orange, or yellow options—while larvae of this species may prefer blue and white surfaces (Kawamura et al., 2016). Further research is needed to better understand the color preferences of shrimp and if this varies across age classes. We also do not know how much welfare shrimp lose from having their color preferences frustrated.
Feed management (domain 1: nutrition)
We examine two welfare threats that can result from feed management12: whether poor feed quality may cause malnutrition and whether feeding frequency may cause hunger. Alongside these, we also discuss whether shrimp are fed the meals they desire most to examine if shrimp feeding preferences may be unmet.
Malnutrition
Artificial shrimp feeds are optimized for growth but this can cause malnutrition and reduce a shrimp’s ability to respond to or resist diseases (see Zhang et al., 2022, pp. 392–401 for review). Other forms of malnutrition that are probably less common, such as lack of protein, can reduce shrimp growth or cause weight loss because protein stored in the body is used for energy for other processes (Zhang et al., 2022, p. 393).
Shrimp feeds were traditionally made from fish oil and meals, but as the aquaculture industry grew, fish-derived feeds could not meet the required level of supply (Hardy, 2010; National Research Council, 2011). Therefore, fish meal, and consequently shrimp feed, prices have risen (Ayisi et al., 2017; Nunes et al., 2022, Figure 1). Feeds are instead increasingly produced using terrestrial farmed animal meat and plant-based (usually soy) meal and oils (Aaqillah-Amr et al., 2021; Naylor et al., 2021), which does not show impacts on shrimp growth (McLean et al., 2020). While evidence about the effects of soy is scarce, this broodstock company website suggests that P. vannamei shrimp need to be selectively bred to tolerate high levels of soybean meal. Plant-based feeds may also lack some important minerals that shrimp require (Zhou et al., 2022, p. 384). It is, therefore, unclear what welfare effects may be caused by the industry’s move away from fish meal toward plant-based protein sources. Biofloc can help maintain shrimp growth rates when alternatives to fishmeal are used as feed, due to the supplemental feed it provides (Moreno-Arias et al., 2018; Xu et al., 2012). To ensure shrimp eat artificial feeds, attractants and palatability-enhancers are added to pellets. These often include aquatic proteins like squid and krill, because shrimp show a preference for these, even when they confer no benefit on growth (Grey et al., 2009; Nunes et al., 2006; Smith et al., 2005; Suresh et al., 2011).
Providing live feed results in better survival and growth for P. vannamei shrimp because they consume live feed at a higher rate than artificial feeds, perhaps suggesting they prefer such a diet (Xue et al., 2021). However, feed-to-flesh conversion is lower with live feeds (Xue et al., 2021), and they may pose a cross-species disease transmission risk (Walker & Mohan, 2009), so farmers are potentially unlikely to use this feeding strategy. Available information suggests that the exception is in broodstock, where shrimp are fed live feed because it significantly improves maturation (Chimsung, 2014; see Kona Bay Broodstock Company). Shrimp preferences may not be met when aquatic proteins or live food are not provided, but we are very uncertain about this.
We expect feed quality problems not to be a prevalent issue, as they directly affect the production level farmers can attain, so they are highly incentivized to ensure shrimp are meeting their nutritional requirements. Still, feed is the highest operational cost (Aaqillah-Amr et al., 2021), so shrimp farmers may prefer to use cheaper feeds, which could influence shrimp welfare if they are of lower quality.
Hunger
Shrimp have small digestive tracts, so they graze on the seafloor, eating small quantities almost continually (Jory & Akiyama, 2006, p. 88; Reis et al., 2020). Therefore, they need to be able to feed multiple times per day. However, farmers cannot just add lots of food into the pond at once, allowing shrimp to graze as they like, because nutrients leach quickly (Bardera et al., 2022), and over-enrichment negatively impacts water quality (see Water quality section for more details).
Farmers generally feed shrimp by hand, scattering feed into the ponds. Additionally, to check whether to adjust the feeding regime based on how much the shrimp are eating, farmers use feeding trays that can be lowered into and out of the pond periodically (Reis et al., 2022, p.360). Some farmers forgo hand feeding, instead using many trays distributed across the pond bottom. Some intensive farms use automatic feeders (HATCH, 2019e).
Several studies report improved growth with increasing feeding frequency (e.g., Aalimahmoudi et al., 2016; Ullman et al., 2019a). Increasing feeding frequencies may be costly to farmers if they increase the total feed given. However, even if the same total amount of feed is given, it seems likely that shrimp would benefit from smaller but more frequent quantities. For example, Ullman et al. (2019b) found that increasing feed by 15% but maintaining feeding frequency at twice per day had no effect on shrimp growth, whereas maintaining the same amount of feed but increasing feeding frequency to six times per day improved growth.
When P. monodon are fed more frequently, instances of cannibalism decrease (Abdussamad & Thampy, 1994, p. 70). During experimental starvation periods, penaeids show increased cannibalism (Lara et al., 2017), and freshwater species show increased aggression (Pontes et al., 2020). Abdussamad and Thampy (1994, p. 70) suggest that for P. monodon feeding six times a day would make cannibalism nearly nonexistent. Starved shrimp also exhibit reduced immune function (Dai et al., 2018). There are mixed results on whether feeding frequency influences P. vannamei survival (Aalimahmoudi et al., 2016; Carvalho & Nunes, 2006). Pedrazzani et al. (2023, Table 12) recommend at least two feeds per day in the ongrowing stage (and at least 4 feeds per day when shrimp weigh less than 0.9g) and at least six feeds per day in the larval and postlarval stages.
Reis et al. (2022, p. 361) report that general practices include feeding shrimp two to four times a day, over eight to twelve hours. Recent surveys conducted in Thailand and Vietnam found that P. vannamei shrimp are usually fed five times per day (Boyd et al., 2017), whilst, in India, the same species is typically fed two to five times per day (Boyd et al., 2018). As with other welfare threats, it was difficult to find more specific data on feeding frequency practices at farms, limiting the inferences we can draw about the prevalence of welfare threats from underfeeding. Available aquaculture research is not sufficient for determining what the optimal feeding frequency for improved shrimp welfare is. Possibly, feeding shrimp four to six times a day can eliminate a lot of welfare issues, but we do not know how much the optimal feeding frequency varies with species, farm type, and feed.
Finally, shrimp are probably not fed at night, due to difficulties with having employees work in these periods (Reis et al. (2022, p. 361). While this probably is not affecting farm productivity, it may be harming shrimp welfare because farmed shrimp are nocturnal (FAO, 2023c; Santos et al., 2016). While shrimp will search for food in both light and dark phases (Pontes et al., 2006), it is possible they have a preference for a feeding regime that more closely matches their nocturnal activity patterns.
Predators (domain 4: behavior)
Shrimp in outdoor farms may be subject to predation by birds. Ponds using natural water sources may also allow entry by aquatic predators. The highest rate of mortality from predation in the wild is probably when shrimp are small, in the larval and postlarval stages (Dall et al., 1991, p. 357).
Insects, like dragonflies, carnivorous fish, and birds are the most prevalent predators of Macrobrachium shrimp (Valenti et al., 2009a, pp. 164–166). For penaeids, predators include cephalopods, crabs, and various fish species (Dall et al., 1991, pp. 359-365). Diseased shrimp are also probably more vulnerable to predation (Gooding et al., 2020). Given that shrimp are adapted to respond to high predation pressure in the wild, even when farmed shrimp are not killed by predators, they are likely stressed by their presence.
We were unable to find estimates of the incidence of predators or data on how much mortality predators typically cause. But, in general, farmed shrimp are subject to less predation pressure than they face in their natural environments (Minello et al., 1989; Salini et al., 1990). Predation is probably mostly a problem for shrimp raised in extensive systems. Fast (1992, p.346) states that one of the main causes of premature deaths of penaeids in extensive ponds is "the high incidence of unwanted predators and competitors.” Macrobrachium shrimp are typically reared in less intensive facilities than penaeids, so may face higher predation pressure.
If predator and competitor animals are found in ponds, they are typically eliminated by draining. Farmers may also use scarecrows, hanging ribbons, or netting to deter birds (HATCH, 2019f). Mesh screens can be used at water inlets to prevent aquatic predators' entry (Alune, 2020; Apud et al., 1983, p.13). Farmers also employ gillnets and poisons that kill predators but not shrimp (see Terazaki et al., 1980). In general, we expect that farmers employ methods to deter predators wherever possible, but we are unsure how successful they are.
Eyestalk ablation (domain 3: health)
As the industry began breeding shrimp in captivity, it found that female maturation was slow and spawning was unpredictable. Eyestalk ablation was initially used to compensate for an inadequate understanding of the environmental, physical, and nutritional factors required for maturation (Bray & Lawrence, 1992, p. 93–94; Treece & Fox, 1993, p. 70). However, farmers often still ablate breeders, even when conditions are adequate for quick maturity. This may be explained by the greater predictability eyestalk ablation offers, as it is very likely that females will spawn shortly after ablation (Benchmark Insights, 2021, p. 17; FAO, 2003, p. 21; Treece & Fox, 1993, p. 70).
In simple terms, this procedure consists of slicing or cutting off one of a female shrimp's eyestalks. In shrimp, as is true of other crustaceans, a hormone responsible for inhibiting ovary maturation is primarily synthesized in the X-organ sinus gland, which is situated in the eyestalk (Kang et al., 2014; Quackenbush, 2001). Ablation is thought to reduce this gonad-inhibiting hormone (also known as vitellogenesis-inhibiting hormone)13 and thus accelerate maturation (Bauer, 2023, p.628; Ogle, 1992; Primavera, 1989; Wickins & Lee, 2002, pp. 16–17). Various techniques are used to ablate a shrimp’s eyestalk (Table 5); pinching is the most common because it is the simplest and cheapest (EGTOP, 2014, pp. 15–16; Primavera, 1989, pp. 11–12).
Table 5: Eyestalk ablation techniques. Based on Bray & Lawrence (1992, pp. 118–119), EGTOP (2014, pp. 15–16), and Primavera (1989, pp. 11–12).
Indicators of pain
After having one eyestalk ablated, shrimp display several behaviors potentially indicative of pain, like tail-flicking (see Box 2), recoiling, stooping (lying prone on the pond floor), disorientation-indicative behaviors, avoiding shelter, erratic swimming, and rubbing the affected area for a period of time after ablation (Barr et al., 2008; Diarte-Plata et al., 2012; Taylor et al., 2004). In particular, Diarte-Plata et al. (2012) report that ligation causes more stress than other techniques, as indicated by significantly more tail-flicking, recoil, disorientation, and rubbing of the affected area. Additionally, up to 80% of the studied shrimp (Macrobrachium americanum) displayed similar behaviors when a slitting ablation method was used. Similarly, in Taylor et al.’s (2004) study, 80% of the shrimp (P. vannamei) exhibited erratic swimming behavior right after the animals had one of their eyes ablated. Shrimp show fewer behavioral signs of stress if a topical anesthetic is applied before ablation (Diarte-Plata et al., 2012; Taylor et al., 2004). Pinching, the most commonly used method, is likely to cause more prolonged pain than the other methods.
Some argue that not all breeders respond to ablation with behaviors like tail-flicking or startling (e.g., Diggles, 2019; A. J. Ray, personal communication, April 14, 2020). The European Union’s Expert Group for Technical Advice on Organic Production also writes that “ablation appears to be a relatively minor discomfort” (EGTOP, 2014, p. 16). We are unsure how much stock to put in these claims, as we did not find published evidence that speaks to the frequency of negative reactions to eyestalk ablation. As explained in Box 2, It could also be that behaviors that seem to indicate pain are either reflexive or caused by something else—for example, an ablated shrimp may swim erratically simply because its vision is impaired (Weineck et al., 2018).
Health implications
The eyestalk of shrimp is also the center for endocrine activity for functions beyond maturation. Disturbance to the endocrine system may explain why ablation increases mortality rates of broodstock by around 30% within 45 days (de Menezes et al., 2019; Magaña-Gallegos et al., 2018; Sainz-Hernández et al., 2008). Ablation disrupts molt cycles and immune responses, and increases energetic demands due to more frequent egg production (Benzie, 1998; Browdy & Samocha, 1985; de Menezes et al., 2019; also see Table 1 in Albalat et al., 2022).
Ablation of breeder shrimp also negatively affects their offspring. In particular, these shrimp show less resistance to common diseases (Zacarias et al., 2021). As shrimp spawn several thousand offspring at once (FAO, 2009a; 2009b; 2023c), diminished immunity of offspring from eyestalk ablation could be a widespread welfare threat.
Handling and transportation (domains 2, 3: environment, health)
Oxidative stress
Air exposure results in oxidative stress. In P. vannamei, death is practically inevitable beyond 30 minutes of air exposure at their normal metabolic rate (Liu et al., 2015), whereas other farmed penaeids show substantial mortality rates after a few hours (Duan et al., 2016a; Duan et al., 2016b). Signs of potential distress include "manic behavior, frequent jumping, abdominal appendage motion, branchial motion, and increased heartbeat," which began immediately after air exposure (Liu et al., 2015, p. 44).
Oxidative stress occurs when transferring shrimp via a net from one pond or tank to another. It can also occur in long-range waterless transport if farmers do not adhere to best practices. Lowering shrimp's metabolic rate should reduce oxidative stress, but the temperature reduction while they are still in water should happen slowly, around four hours for P. monodon (Salin & Jayasree-Vadhyar, 2001). Reducing temperature over 30 minutes, apparently common in practice, causes oxidative stress which can drastically increase mortality (Xu et al., 2021, p. 8). We believe that only adults are subjected to long-range waterless transport—domesticated broodstock and shrimp who will be sold alive at market.
Handling Stress
Handling risks physical damage and stress. Physical damage could occur from being held too firmly by farmers or incidental contact with other shrimp or objects. For example, when shrimp are collected by net, they can be "pressed against the end of the net" if dragged too quickly. Villalón (1991, p. 41) recommends ensuring individuals are at the intermolt stage so that they can withstand any physical damage that might occur. Farmers likely do their best, but given individual differences in growth some shrimp will be molting during transportation even when timing is optimal.
The handling process, while not intended to cause harm, may be interpreted by shrimp as a lethal threat. For example, they might process nets chasing them the same way as they respond to predators, or process inspection outside of the tank the same way as being washed up ashore. Consistent with these possibilities, both chasing and air exposure cause a primary stress response (Aparicio-Simón et al., 2010; Shinji et al., 2012). That said, beyond anecdotes such as delayed reengagement in feeding, we do not know what the psychological effects are of handling stress.
Pumping shrimp between ponds allows them to stay in water the entire time, and so may cause the least handling stress. Ohs et al. (2007) claim that they "have successfully transferred 400,000 juvenile shrimp over a horizontal distance of 200 feet (61 m) in less than two hours without any observed mortality or damage to the shrimp." It is unclear whether this statement only holds under optimal conditions or whether they would choose to report negative outcomes that do not meaningfully affect farmers' profits. Also, it is possibly stressful for shrimp to be suddenly pulled by a strong current to an unknown location.
High Stocking Density
During water-based transportation, the number of individuals per liter is a function of shrimp age, size, shipping conditions, and shipment duration (Olin & Fast, 1992, p. 310). Broadly, the smaller the shrimp the higher the densities, but the longer the shipment duration the lower the densities to avoid adverse effects on water quality. For freshwater species, postlarvae "are about 7 mm long and begin to swim and crawl like adult animals...Two thousand PL [postlarvae] are allocated to each plastic packing bag (0.3 × 0.4 m) containing 3 L of water" (Valenti et al., 2009b, pp. 78–79). Each individual will have about 1.5 cm3 to itself, more than enough room to turn around in any direction. Similarly, Saputra (2022, p. 2) reported an industry standard of 2,000 P. vannamei shrimp/45,000 mL when transporting postlarvae from hatcheries to farms within Indonesia. The authors imply that shrimp who have been postlarvae for 10 days is a standard age for transportation. If shrimp at this age are at most 0.5 cm long (Teixeira & Guerrelhas, 2014), they should have ample room to themselves. Limited mobility per se is probably not a common issue when transporting postlarvae, although it could be that they would prefer more space.
High stocking densities likely exacerbate other transportation issues though. An FAO (2007, p. 75) manual focused on P. monodon recommends a stocking density of 250–500 postlarvae per liter for transportation from the hatchery to ongrowing harm. They do not specifically justify this range, but the same section mentions strategies for limiting cannibalism, high ammonia, and low dissolved oxygen, which are more common at higher densities (Saputra et al., 2022; Sasikumar & Vadhyar, 1998). Debnath et al., (2016, Table 3) reported densities of 4,000 to 5,000 P. monodon postlarvae in 3-liter bags for a 4–5 hour trip within Bangladesh, much higher than FAO's recommendations. However, we do not know whether these practices generalize beyond Bangladesh.
Poor Water Quality
Water quality during preparation for transportation worsens due to disruption of the benthic environment. Catching shrimp using a net "requires that farm personnel walk in the pond, which results in a high degree of sediment suspension that may foul gills and cause undue stress to the juveniles" (Villalón, 1991, p.41). Similarly, Ohs et al. (2007) note that the last shrimp who are transferred by pumping may have "prolonged exposure to concentrated sediments." These issues could affect any shrimp being transferred between earthen ponds.
During transportation itself, water quality issues arise due to a buildup of ammonia and consumption of dissolved oxygen. Huang et al., (2023) found that after a simulated 72-hour transportation, survival rates for P. vannamei shrimp were 65% when no water was exchanged, and 94% when water was exchanged, and that levels of un-ionized ammonia were significantly higher in the no-water-exchange group.
Farmers normally have an incentive to keep water quality acceptable during transportation. A partial exception comes from Debnath et al. (2016; see also Meshram et al., 2009, p. 186), who note that competition for wild-sourced broodstock supply among hatcheries allows trawlers to lower their transportation standards:
|
[W]ater is no longer aerated using compressed oxygen during transportation of broodstock to hatcheries after landing. This was a common practice in the past, but is no longer followed widely as lack of aeration does not result in immediate mortality. This has led poorly educated hatchery staff to believe that oxygenation makes little difference to broodstock condition, while in fact, failure to oxygenate water during transport is likely to place additional stress on recently captured broodstock (p. 4) |
|---|
As an illustration of the potential welfare consequences, eyestalk ablation has been largely discontinued in Bangladesh, as it causes already-weakened broodstock to die before spawning (Debnath et al., 2016). The supply chain for wild-caught postlarvae to farms persists despite bans on their collection in countries like Ecuador and Bangladesh (Ahamed et al., 2012; Páez-Osuna, 2001), but may be on the decline as the industry modernizes.
Duration of Transportation
Hatcheries and their customers often reside in the same country. Within Bangladesh, Debnath et al. (2016, Table 3) report transportation times between 4 and 28 hours. Industry researchers often report survival rates of "up to 80 percent" after 12 hours or even a whole day (Goodrick et al., 1993; Xu et al., 2021). Best management practices can achieve over 95% survival within 24 hours, but "moderate to poor survival" after that (Flick & Kuhn, 2015; see also Salin & Jayasree-Vadhyar, 2001; Skudlarek et al., 2011). Survival in actual practice is potentially worse than in study conditions where researchers are dealing with a comparatively small number of animals and using optimal water quality conditions in the post-transport tanks. Moreover, the survival rate upon arrival does not necessarily account for the total percentage that will die in the following days due to the tumultuous journey.
The longest transport time we have seen reported is 72 hours (e.g., Benchmark Genetics Shrimp, 2020). Domesticated broodstock sold by suppliers probably experience the longest transportation times on average. About 85% of first-generation P. vannamei broodstock are imported from other countries, mostly the United States (Shrimp Insights, 2020, p. 32). Florida-based American Penaeid, Inc. boasts of being within three and a half hours away from international airports. Their customers are 8–28 hours away by plane. If we assume hatcheries are also about 3.5 hours away from international airports, then the total shipment time is 15–35 hours in total. An operator we called at American Penaeid reported an ~85% survival rate for broodstock.
Harvest (domains 2, 3: environment, health)
Harvest (collecting shrimp out of the pond) is a form of handling and transportation, so all of the same stressors are present. One common indicator of stress during harvest is jumping out of the pond (Pedrazzani et al., 2023), as can be seen in this video. Electro-fishing for catching shrimp purposely makes shrimp jump, which might make it the least humane harvest method. On the other hand, jumping, like tail-flicking, might be a reflex that does not involve conscious processing.
Harvest also poses unique welfare threats in virtue of the fact that it is the last form of handling before slaughter. For example, Lucien-Brun (2016a) recommends suspending feed for four to six hours before harvesting them. This is primarily to prevent effects on color that customers do not like (Otwell et al., 2001). Apparently, the standard in the past was to stop feeding for at least 48 hours. Given our earlier observation that shrimp normally eat frequently, the sudden cessation of feed may make shrimp hungry and frustrated. Indeed, Lucien-Brun (2016a) observes that "If no formulated feed is available because feeding was suspended, the shrimp will burrow into the pond bottom sediment in search of food." Burrowing "will give them an unattractive appearance" so farmers have an incentive to wait longer to suspend feed, but we are unsure how many farmers are familiar with best practices in this area.
Harvest is also less welfare-friendly than other forms of handling and transportation because farmers no longer need to keep the shrimp alive. For example, during waterless transportation, farmers cool shrimp to minimize oxidative stress that could kill them. During harvest, though, shrimp are not necessarily slaughtered right away. Instead, they endure air exposure at ambient temperature while farmers wash, weigh, and sort them (FAO, 2009a). This waiting period can be prolonged. Shrimp Welfare Project's (2022, p.15) visits to farms in India revealed that "once shrimp are taken from the water via a dragnet, they are placed in crates and left for several minutes before being weighed." Shrimp who are discarded (e.g., because of discoloration) likely die from air exposure as they never make it to slaughter. Even shrimp who will be sold may die during this evaluation period, as death from oxidative stress only takes a bit longer than 30 minutes for P. vannamei (Liu et al., 2015). This video shows shrimp being gathered in a large collection net, scooped out using a crate and placed into smaller, tightly packed mesh bags out of the water. These are then carried to another location, and the shrimp are emptied into plastic crates. Only a few shrimp are still seen to be moving at this point; possibly, the rest died from prolonged air exposure. On the other hand, we also found one video showing shrimp being stunned almost immediately (see the Slaughter section below), so it is not clear how common prolonged harvest processes are.
Finally, some shrimp may die neither from slaughter nor air exposure, but from being crushed by other shrimp. In the first video linked above (watch from 7:12) they are stored in overcrowded sacks with no ice. Shrimp Welfare Project (2022, p.15) observed shrimp "being crushed both by other shrimp and by workers (who step on the shrimp when placing another net full of shrimp into remaining crates)." Mechanical pumps are probably less likely to damage shrimp during harvest (Ohs et al., 2006). Farmers should have an incentive to avoid crushing because shrimp with gross injuries may not be able to be sold. However, some are paid by the quantity of shrimp and their average weight (Lucien-Brun, 2016a), not based on product quality.
Slaughter (domains 2, 3: environment, health)
In this section, "stunning" refers to rendering an individual insensible. The point beyond which stunning is irreversible is considered slaughter. Electrical stunning is beginning to gain popularity (Compassion in World Farming, n.d.), but we do not discuss its humaneness here because it is still a novel technique that has not yet been widely adopted or researched. Instead, we focus on ice slurry slaughter, which we believe is by far the most common stunning technique. The slurry's low temperature (typically below 4ºC/39.2°F; Lucien-Brun, 2016b; Piana et al., 2018) is believed to minimize the pain of death (EFSA, 2005, p. 32; RSPCA Australia, 2019, pp. 2–4) and improve food safety (Bothén, 2015).
Crustaceans exhibit few behavioral signs of distress during the process of chilling, but their nervous systems could still be functional even if they are unable to move (Conte et al., 2021, p. 6). Unfortunately, evidence about how quickly ice slurry renders shrimp insensible or kills them is scarce. To measure stunning, Weineck et al. (2018) observed six P. vannamei shrimp’s physiological responses to ice slurry. After 30 seconds, the shrimp showed no response to external stimuli. Upon return to warm seawater, these responses were less pronounced than before the cold shock (p. 10). The authors concluded that ice slurry successfully reduced nociception. In contrast, for M. rosenbergii, paralysis after a rapid reduction in water temperature from 21˚C to 5˚C took about 20 minutes (Chung et al., 2012, p. 79).
For time to death, Chung et al., (2012) reported that "after an hour [M. rosenbergii are] on their side completely flaccid. After placing them back in water at 21˚C they did not recover" (p. 79). Weineck et al. (2018) observed that P. vannamei's heart rate rapidly decreased upon submersion in the ice slurry, with no detectable heartbeat by four minutes at most. The heartbeat of shrimp is controlled by the cardiac ganglion, which contains interneurons that can modulate heart activity based on information from other neurons (Cooke, 2002). Weineck et al. (2018) argue that the cessation of heart rate therefore indicates a lack of cognition (p. 4). Even if this is true, ice slurry stunning is at best reversible, as the shrimp's heart rates increased upon returning to warm seawater, save for one individual who died in the ice slurry. We are unsure how likely recovery is in practice, though, as shrimp are transported to processors in iced bins (FAO, 2009a).
Unfortunately, neither of these studies provides strong evidence of the latency to stunning and slaughter in commercial settings. Chung et al. (2012) did not remove shrimp from the water first, and the temperature they used is 5˚C higher than is recommended for M. rosenbergii (FAO, 2009b). Weineck et al.’s (2018) procedure was comparatively realistic, but it still placed each shrimp in the slurry individually. On farms, shrimp are placed in slurries en masse, which reduces the likelihood that any one of them is fully immersed.
Weineck et al. (2018) also submerged shrimp in saltwater ice, consistent with guidelines to prevent shrimp from experiencing osmotic shock (RSPCA Australia, 2019, p. 3). A somewhat recent industry source implied that marine shrimp normally are submerged in iced salt water (Piana et al., 2018). On the other hand, a handbook on crustacean aquaculture states that marine shrimp should be “submerged in a chilled ice slurry fresh water solution” (Villalón, 1991, p. 102). Given this contradictory information, we consulted several experts to discern what standard practices are. Three of them stated that water from the production system is usually used to produce the ice slurry.14 However, a fourth expert stated that freshwater is commonly used for the ice slurry, apparently regardless of the shrimp species in question. Another expert confirmed our earlier observation that many farmers dump shrimp directly into a container with ice only. The lack of consensus suggests that different producers have different procedures. Even if water from the production system is used to produce the ice slurry, two aquaculture experts informed us that the ice itself is provided by the processing plant and must be made from potable freshwater to comply with food safety regulations (personal communication, Elena Piana, April 12, 2023). Therefore, the progressive melting of the ice will lower the water salinity level. However, it might be that salinity levels do not reach a painful level until the animals are already stunned or dead. Additionally, some producers add salt to the slurry to quickly lower its temperature and delay ice melting (Lucien-Brun, 2016b). This may help maintain the salinity of the ice slurry and probably contributes to quicker stunning.
Finally, there is uncertainty about how much distress shrimp experience from cold shock before they are fully stunned. Hyperglycemia occurs when M. rosenbergii are transferred directly from their optimal temperature (28 °C) to lower temperatures (Kuo & Yang, 1999). If shrimp are sentient, physiological stress responses probably do result in negative experiences. Fortunately, the pain might not last long, as heart rate rapidly decreases upon cold shock, which Weineck et al. (2018) interpret as evidence of stunning. However, we are unsure whether information about temperature is conveyed to the cardiac ganglion via the brain or directly from sensory neurons. In the latter case, it could be that the brain activity responsible for pain continues unabated even when heart rate decreases. The only other potential indicator of pain we are aware of is tail-flicking vigorously upon submersion in the ice slurry (Weineck et al., 2018). As discussed earlier though, tail-flicking is reflexive and we are unsure whether the nociception that causes it also results in pain.
Conclusion
Farmed shrimp appear to face a wide variety of threats, ranging from cannibalism to toxic levels of ammonia to suffocation. Because farmed shrimp are so numerous, these issues constitute an enormous welfare loss in the aggregate, assuming shrimp are sentient (Waldhorn & Autric, 2023). Given uncertainty about shrimp sentience, the risk of serious, negative outcomes leads us to conclude that the Animal Sentience Precautionary Principle indeed applies to shrimp aquaculture (Birch, 2017). The implication is that stakeholders should seek to improve the welfare of farmed shrimp now, in parallel with additional research on shrimp sentience.
Future reports in the Shrimp Welfare Sequence will translate this call to action into a more specific plan. In particular, we will lay out existing efforts to help farmed shrimp, as well as additional strategies that have not been tried yet. To help prioritize among possible actions, we will estimate how much pain each welfare issue identified here causes in the aggregate. For example, some issues affect small subpopulations of farmed shrimp only, such as eyestalk ablation, while others affect nearly all farmed shrimp when they are present, such as poor water quality. We will also examine how the preslaughter mortality rate varies across the course of production, which might help determine when it is most crucial to intervene. Finally, our ability to learn about the harms of shrimp aquaculture was constrained by a dearth of research that investigates the topic from a welfare perspective.
Acknowledgments
This report is a project of Rethink Priorities—a think tank dedicated to informing decisions made by high-impact organizations and funders across various cause areas. The authors are Hannah McKay, William McAuliffe, and Daniela Waldhorn. Thanks to Partho Debnath, Holly Elmore, Bella Forristal, Elena Piana, Andrew Ray, and other reviewers for helpful feedback on drafts of the report, and to Adam Papineau for copy-editing. Thanks to Shrimp Welfare Project for providing raw data and general insights.
If you are interested in RP’s work, please visit our research database and subscribe to our newsletter.
Bibliography
Aalimahmoudi, M., Reyshahri, A., Bavarsad, S. S., & Maniat, M. (2016). Effects of feeding frequency on growth, feed conversion ratio, survival rate and water quality of white leg shrimp (Litopenaeus vannamei, Boone, 1931). International Journal of Fisheries and Aquatic Studies, 4(3), 293–297. https://perma.cc/Z3ZU-8A47
Aaqillah-Amr, M. A., Hidir, A., Azra, M. N., Ahmad-Ideris, A. R., Abualreesh, M. H., Noordiyana, M. N., & Ikhwanuddin, M. (2021). Use of Pelleted Diets in Commercially Farmed Decapods during Juvenile Stages: A Review. Animals, 11(6), 1761. https://doi.org/10.3390/ani11061761
Abdussamad, E. M., & Thampy, D. M. (1994). Cannibalism in the tiger shrimp Penaeus monodon Fabricius in nursery rearing phase. Journal of Aquaculture in the Tropics, 9(1). https://perma.cc/7T84-XFD5
Ahamed, F., Hossain, Md. Y., Fulanda, B., Ahmed, Z. F., & Ohtomi, J. (2012). Indiscriminate exploitation of wild prawn postlarvae in the coastal region of Bangladesh: A threat to the fisheries resources, community livelihoods and biodiversity. Ocean & Coastal Management, 66, 56–62. https://doi.org/10.1016/j.ocecoaman.2012.05.025
Albalat, A., Zacarias, S., Coates, C. J., Neil, D. M., & Planellas, S. R. (2022). Welfare in Farmed Decapod Crustaceans, With Particular Reference to Penaeus vannamei. Frontiers in Marine Science, 9. https://doi.org/10.3389/fmars.2022.886024
Alday-Sanz, V., Brock, J., Flegel, T. W., McIntosh, R., Bondad-Reantaso, M. G., Salazar, M., & Subasinghe, R. (2020). Facts, truths and myths about SPF shrimp in Aquaculture. Reviews in Aquaculture, 12(1), 76–84. https://doi.org/10.1111/raq.12305
Allan, G. L., & Maguire, G. B. (1991). Lethal levels of low dissolved oxygen and effects of short-term oxygen stress on subsequent growth of juvenile Penaeus monodon. Aquaculture, 94(1), 27–37. https://doi.org/10.1016/0044-8486(91)90126-R
Allan, G. L., Maguire, G. B., & Hopkins, S. J. (1990). Acute and chronic toxicity of ammonia to juvenile Metapenaeus macleayi and Penaeus monodon and the influence of low dissolved-oxygen levels. Aquaculture, 91(3), 265–280. https://doi.org/10.1016/0044-8486(90)90193-Q
Alune. (2020). Top 10 tips for shrimp farming – the basics. The Fish Site. https://perma.cc/JM4L-T5UC
Alune. (2021a). Exploring the use of probiotics in shrimp farms. The Fish Site. https://perma.cc/GM56-68E2
Alune. (2021b). Seven tips to improve pond bottom quality in shrimp farms. The Fish Site. https://perma.cc/8Y3J-9VLF
Anand, P. S. S., Balasubramanian, C. P., Christina, L., Kumar, S., Biswas, G., De, D., Ghoshal, T. K., & Vijayan, K. K. (2019). Substrate based black tiger shrimp, Penaeus monodon culture: Stocking density, aeration and their effect on growth performance, water quality and periphyton development. Aquaculture, 507, 411–418. https://doi.org/10.1016/j.aquaculture.2019.04.031
Anderson, J. L., Valderrama, D., & Jory, D. E. (2016a). GOAL Shrimp Production Survey: Recovery coming. Global Seafood Alliance. https://perma.cc/FF3X-4LW4
Anderson, J. L., Valderrama, D., & Jory, D. E. (2016b). Global shrimp survey: GOAL 2016. Global Seafood Alliance. https://perma.cc/EH83-VD8G
Anderson, J. L., Valderrama, D., & Jory, D. E. (2017). GOAL 2017: Global shrimp production review and forecast. Global Seafood Alliance. https://perma.cc/4F43-4F74
Anderson, J. L., Valderrama, D., & Jory, D. E. (2018). Global shrimp production review and forecast: Steady growth ahead. Global Seafood Alliance. https://perma.cc/L3TD-3RKJ
Anderson, J. L., Valderrama, D., & Jory, D. E. (2019). GOAL 2019: Global shrimp production review. Global Seafood Alliance. https://perma.cc/A5N7-TZHL
Aparicio-Simón, B., Piñón, M., Racotta, R., & Racotta, I. S. (2010). Neuroendocrine and metabolic responses of Pacific whiteleg shrimp Litopenaeus vannamei exposed to acute handling stress. Aquaculture, 298(3–4), 308–314. https://doi.org/10.1016/j.aquaculture.2009.10.016
Apud, F., Primavera, J. H., & Torres, P. L. (1983). Farming of prawns and shrimps (Aquaculture extension manual No. 5) (3rd ed.). Tigbauan, Iloilo, Philippines: SEAFDEC Aquaculture Department. https://perma.cc/R7DA-LHLP
Arala-Chaves, M., & Sequeira, T. (2000). Is there any kind of adaptive immunity in invertebrates? Aquaculture, 191(1), 247–258. https://doi.org/10.1016/S0044-8486(00)00430-0
Araneda, M., Pérez, E. P., & Gasca‐Leyva, E. (2008). White shrimp Penaeus vannamei culture in freshwater at three densities: Condition state based on length and weight. Aquaculture, 283(1–4), 13–18. https://doi.org/10.1016/j.aquaculture.2008.06.030
Arnott, S. A., Neil, D. M., & Ansell, A. D. (1998). Tail-Flip mechanism and Size-Dependent kinematics of escape Swimming in the brown shrimp crangon crangon. The Journal of Experimental Biology, 201(11), 1771–1784. https://doi.org/10.1242/jeb.201.11.1771
Asche, F., Anderson, J. L., Botta, R., Kumar, G., Abrahamsen, E. B., Nguyen, L. T., & Valderrama, D. (2021). The economics of shrimp disease. Journal of Invertebrate Pathology, 186, 107397. https://doi.org/10.1016/j.jip.2020.107397
Ayisi, C. L., Hua, X., Apraku, A., Afriyie, G., & Kyei, B. A. (2017). Recent Studies Toward the Development of Practical Diets for Shrimp and Their Nutritional Requirements. HAYATI Journal of Biosciences, 24(3), 109–117. https://doi.org/10.1016/j.hjb.2017.09.004
Balasundaram, C., Jeyachitra, P., & Balamurugan, P. (2004). Shelter preference in Macrobrachium spp. With reference to aquaculture. Acta Ethologica, 7(2), 95–101. https://doi.org/10.1007/s10211-004-0090-4
Bardera, G., De, J. B. D., Tailly, M. D. F. A., & Pontes, C. S. (2022). Shrimp Feeding Behaviour. The Shrimp Book II. (pp. 336–356). 5m Books Ltd.
Bardera, G., Owen, M. A. G., Façanha, F. N., Alcaraz-Calero, J. M., Alexander, M. E., & Sloman, K. A. (2021). The influence of density and dominance on Pacific white shrimp (Litopenaeus vannamei) feeding behaviour. Aquaculture, 531, 735949. https://doi.org/10.1016/j.aquaculture.2020.735949
Barg, U., Subasinghe, R., Willman, R., Rana, K., & Martinez, M. (1999). Towards Sustainable Shrimp Culture Development: Implementing the FAO Code of Conduct for Responsible Fisheries (CCRF). https://perma.cc/5N9A-NE43
Barr, S., Laming, P. R., Dick, J. T. A., & Elwood, R. W. (2008). Nociception or pain in a decapod crustacean? Animal Behaviour, 75(3), 745–751. https://doi.org/10.1016/j.anbehav.2007.07.004
Bauer, R. T. (2004). Remarkable Shrimps: Adaptations and Natural History of the Carideans. University of Oklahoma Press.
Bauer, R. T. (2010). Chemical Communication in Decapod Shrimps: The Influence of Mating and Social Systems on the Relative Importance of Olfactory and Contact Pheromones. In T. Breithaupt & M. Thiel (Eds.), Chemical Communication in Crustaceans (pp. 277–296). Springer. https://doi.org/10.1007/978-0-387-77101-4_14
Bauer, R. T. (2023). Fisheries and Aquaculture. In Shrimps: Their Diversity, Intriguing Adaptations and Varied Lifestyles (pp. 583-655). Springer Cham.
Bautista-Covarrubias, J. C., Valdez-Soto, I. E., Aguilar-Juárez, M., Arreola-Hernández, J. O., Soto-Jiménez, M. F., Soto-Rodríguez, S. A., López-Sánchez, J. A., Osuna-Martínez, C. C., & Frías-Espericueta, M. G. (2022). Cadmium and copper mixture effects on immunological response and susceptibility to Vibrio harveyi in white shrimp Litopenaeus vannamei. Fish & Shellfish Immunology, 129, 145–151. https://doi.org/10.1016/j.fsi.2022.08.054
Benchmark Genetics Shrimp. (2020). Shrimp Broodstock Acclimatisation Procedures. https://perma.cc/M8ZB-QJBD
Benchmark Insights. (2019). Technologies shaping the future of shrimp production. Benchmark. https://perma.cc/KA9P-GV2M
Benchmark Insights. (2021). Welfare in Aquaculture: Drivers, trends and best practice (Issue 2). Benchmark. https://perma.cc/UP6X-6472
Benzie, J. A. H. (1998). Penaeid genetics and biotechnology. Aquaculture, 164(1), 23–47. https://doi.org/10.1016/S0044-8486(98)00175-6
Birch, J. (2017). Animal sentience and the precautionary principle. Animal Sentience, 2(16). https://doi.org/10.51291/2377-7478.1200
Boopathy, R., Bonvillain, C., Fontenot, Q., & Kilgen, M. (2007). Biological treatment of low-salinity shrimp aquaculture wastewater using sequencing batch reactor. International Biodeterioration & Biodegradation, 59(1), 16–19. https://doi.org/10.1016/j.ibiod.2006.05.003
Bothén, K. (2015). Food safety in land-based shrimp productions—Establishment of a HACCP plan at VegaFish AB [Master’s thesis, Sveriges lantbruksuniversitet]. https://perma.cc/D58H-R6FB
Boyd, C. E. (1989). Water quality management and aeration in shrimp farming. https://perma.cc/HJL4-EWK8
Boyd, C. E. (2017a). The inevitable pH fluctuations of aquaculture pond water. Global Seafood Alliance. https://perma.cc/65NT-LX6Z
Boyd, C. E. (2017b). Lime plays crucial role in aquaculture pond management. Global Seafood Alliance. https://perma.cc/2NMZ-64CT
Boyd, C. E., & Fast, A. W. (1992). Pond Monitoring and Management. In A. W. Fast & L. J. Lester (Eds.), Marine Shrimp Culture: Principles and Practices (pp. 497–513). Elsevier. https://doi.org/10.1016/B978-0-444-88606-4.50029-1
Boyd, C. E., & Jescovitch, L. N. (2020). Penaeid Shrimp Aquaculture. In G. Lovrich & M. Thiel (Eds.), Fisheries and Aquaculture: Volume 9. Oxford University Press. https://doi.org/10.1093/oso/9780190865627.003.0010
Boyd, C. E., & Tucker, C. S. (1998). Pond Aquaculture Water Quality Management. Springer New York. https://doi.org/10.1007/978-1-4615-5407-3_3
Boyd, C. E., & Zimmermann, S. (2010). Grow-out Systems – Water Quality and Soil Management. In M. B. New, W. C. Valenti, J. H. Tidwell, L. R. D’Abramo, & M. N. Kutty (Eds.), Freshwater Prawns: Biology and Farming. John Wiley & Sons. https://doi.org/10.1002/9781444314649.ch13
Boyd, C. E., Davis, R. P., & McNevin, A. A. (2022). Perspectives on the mangrove conundrum, land use, and benefits of yield intensification in farmed shrimp production: A review. Journal of the World Aquaculture Society, 53(1), 8–46. https://doi.org/10.1111/jwas.12841
Boyd, C. E., Davis, R. P., Wilson, A. G., Marcillo, F., Brian, S., & McNevin, A. A. (2021). Resource use in whiteleg shrimp Litopenaeus vannamei farming in Ecuador. Journal of the World Aquaculture Society, 52(4), 772–788. https://doi.org/10.1111/jwas.12818
Boyd, C. E., McNevin, A. A., Davis, R. P., Godumala, R., & Mohan, A. B. Ch. (2018). Production Methods and Resource Use at Litopenaeus vannamei and Penaeus monodon Farms in India Compared with Previous Findings from Thailand and Vietnam. Journal of the World Aquaculture Society, 49(3), 551–569. https://doi.org/10.1111/jwas.12524
Boyd, C. E., McNevin, A. A., Racine, P., Tinh, H. Q., Minh, H. N., Viriyatum, R., Paungkaew, D., & Engle, C. (2017). Resource Use Assessment of Shrimp, Litopenaeus vannamei and Penaeus monodon, Production in Thailand and Vietnam. Journal of the World Aquaculture Society, 48(2), 201–226. https://doi.org/10.1111/jwas.12394
Boyd, C.E., Boyd, C.A., & Chainark, S. (2012). The Shrimp Book: Shrimp pond soil and water quality management. Global Seafood Alliance. https://perma.cc/3D5U-V37C
Bratvold, D., & Browdy, C. L. (2001). Effects of sand sediment and vertical surfaces (AquaMatsTM) on production, water quality, and microbial ecology in an intensive Litopenaeus vannamei culture system. Aquaculture, 195(1), 81–94. https://doi.org/10.1016/S0044-8486(00)00538-X
Bray, W. A., & Lawrence, A. L. (1992). Reproduction of Penaeus Species in Captivity. In A. W. Fast & L. J. Lester (Eds.), Marine Shrimp Culture (pp. 93–170). Elsevier. https://doi.org/10.1016/B978-0-444-88606-4.50011-4
Broom, D. M. (1991). Animal welfare: concepts and measurement. Journal of Animal Science, 69(10), 4167–4175. https://doi.org/10.2527/1991.69104167x
Browdy, C. L., & Samocha, T. M. (1985). The effect of eyestalk ablation on spawning, molting and mating of Penaeus semisulcatus de Haan. Aquaculture, 49(1), 19–29. https://doi.org/10.1016/0044-8486(85)90187-5
Buike, P. (2018). Rainy season effects on shrimp grow-out ponds. Global Seafood Alliance. https://perma.cc/NQH9-SENT
Burgos-Hernández, A., Zapién, M. G. L., Madrid, M. L. A., Sifuentes, C. O. G., Gil, C. I. M., Burgos, E. C. R., & Olivas, R. R. (2006). Presence of insecticides in shrimp farms adjacent to the sea of cortes: Detection, quantification, and toxicity testing. European Food Research and Technology, 222(3), 380–384. https://doi.org/10.1007/s00217-005-0012-3
Carvalho, E. A., & Nunes, A. J. P. (2006). Effects of feeding frequency on feed leaching loss and grow-out patterns of the white shrimp Litopenaeus vannamei fed under a diurnal feeding regime in pond enclosures. Aquaculture, 252(2–4), 494–502. https://doi.org/10.1016/j.aquaculture.2005.07.013
Chang, Z.-W., Chiang, P.-C., Cheng, W., & Chang, C.-C. (2015). Impact of ammonia exposure on coagulation in white shrimp, Litopenaeus vannamei. Ecotoxicology and Environmental Safety, 118, 98–102. https://doi.org/10.1016/j.ecoenv.2015.04.019
Chen, H.-Y., & Chen, Y.-L. L. (1999). Temperature preferendum of postlarval black tiger shrimp (Penaeus monodon). Marine and Freshwater Research, 50(1), 67–70. https://doi.org/10.1071/MF97236
Chen, J., Zhou, F., Huang, J., Ma, Z., Jiang, S., Qiu, L., & Qin, J. G. (2016). Ammonia and salinity tolerance of Penaeus monodon across eight breeding families. SpringerPlus, 5(1), 171. https://doi.org/10.1186/s40064-016-1878-1
Chimsung, N. (2014). Maturation diets for black tiger shrimp (Penaeus monodon) broodstock: A review. Songklanakarin Journal of Science and Technology, 36(3), 265–273. https://perma.cc/XP6F-U647
Chung, Y. S., Cooper, R. M., Graff, J., & Cooper, R. L. (2012). The acute and chronic effect of low temperature on survival, heart rate and neural function in crayfish (Procambarus clarkii) and prawn (Macrobrachium rosenbergii) species. Open Journal of Molecular and Integrative Physiology, 2(3). https://doi.org/10.4236/ojmip.2012.23011
Clayton, K. A., Ellis, R. P., Wilson, R. W., & Ferrer, A. (2022). Important water chemistry variable for shrimp production. In V. Alday-Sanz (Ed.), The Shrimp Book II (pp. 286–335). 5m Books Ltd.
Compassion in World Farming (n.d.). Tesco and Hilton Seafood – Improving the welfare of whiteleg shrimp (Pennaus vannamei) at harvest. Compassion in World Farming. https://perma.cc/LEV9-YB5D
Conte, F., Voslarova, E., Vecerek, V., Elwood, R. W., Coluccio, P., Pugliese, M., & Passantino, A. (2021). Humane Slaughter of Edible Decapod Crustaceans. Animals, 11(4), Article 4. https://doi.org/10.3390/ani11041089
Cook, H. L., & Rabanal, H. R. (Eds.). (1978a). Harvesting. In Manual on Pond Culture of Penaeid Shrimp. Association of Southeast Asian Nations (ASEAN). https://perma.cc/6HGN-5GH3
Cook, H. L., & Rabanal, H. R. (Eds.). (1978b). Design Criteria Required by Shrimp. In Manual on Pond Culture of Penaeid Shrimp. Association of Southeast Asian Nations (ASEAN). https://perma.cc/76Y6-EGGM
Crump, A., Browning, H., Schnell, A., Burn, C., & Birch, J. (2022). Sentience in decapod crustaceans: A general framework and review of the evidence. Animal Sentience, 7(32). https://doi.org/doi.org/10.51291/2377-7478.1691
da Costa, F. P., Gomes, B. S. F. de F., Pereira, S. D. do N. A., & de Fátima Arruda, M. (2016). Influence of stocking density on the behaviour of juvenile Litopenaeus vannamei (Boone, 1931). Aquaculture Research, 47(3), 912–924. https://doi.org/10.1111/are.12550
da Silveira, L. G. P., Krummenauer, D., Poersch, L. H., Rosas, V. T., & Wasielesky Jr, W. (2020). Hyperintensive stocking densities for Litopenaeus vannamei grow-out in biofloc technology culture system. Journal of the World Aquaculture Society, 51(6), 1290–1300. https://doi.org/10.1111/jwas.12718
Dai, W.-F., Zhang, J.-J., Qiu, Q.-F., Chen, J., Yang, W., Ni, S., & Xiong, J.-B. (2018). Starvation stress affects the interplay among shrimp gut microbiota, digestion and immune activities. Fish & Shellfish Immunology, 80, 191–199. https://doi.org/10.1016/j.fsi.2018.05.040
Dall, W. H. B. J., Hill, B. J., Rothlisberg, P. C., & Sharples, D. J. (1991). Predation on penaeids. In W. H. B. J. Dall, B. J. Hill, P. C. Rothlisberg., & D. J. Sharples (Eds.), The biology of the Penaeidae., 27. http://doi.org/10.1016/S0065-2881(08)60177-7
Dawkins, M. (1977). Do hens suffer in battery cages? Environmental preferences and welfare. Animal Behaviour, 25, 1034–1046. https://doi.org/10.1016/0003-3472(77)90054-9
Dayalan, V., Kasivelu, G., Raguraman, V., & Sharma, A. N. (2022). Studies on temperature impact (sudden and gradual) of the white-leg shrimp Litopenaeus vannamei. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-022-20963-y
de Menezes, T. B. B., Gomes, I. G. R. F., Lucena, H. M. R., Filho, K. P. do N., Araújo, A. L. A. C. de, Facundo, G. de M., Sousa, R. R. de, Oliveira, E. G. de, César, J. de O., & Costa, F. H. F. (2019). Reproductive performance of non-ablated Penaeus vannamei females in a Brazilian commercial hatchery/ Desempenho reprodutivo de fêmeas não abladas de Penaeus vannamei em uma larvicultura comercial brasileira. Brazilian Journal of Development, 5(12). https://doi.org/10.34117/bjdv5n12-388
Debnath, P. P., Khan, S. H., Karim, M., Belton, B., Mohan, C. V., & Phillips, M. (2016b). Review of the history, status and prospects of the black tiger shrimp (Penaeus monodon) hatchery sector in Bangladesh. Reviews in Aquaculture, 8(3), 301–313. https://doi.org/10.1111/raq.12094
Delgado, P., Avnimelech, Y., McNeil, R., Bratvold, D., Browdy, C. L., & Sandifer, P. A. (2003). Physical, chemical and biological characteristics of distinctive regions in paddlewheel aerated shrimp ponds. Aquaculture, 217(1–4), 235–248. https://doi.org/10.1016/s0044-8486(02)00231-4
Diarte-Plata, G., Sainz-Hernández, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., & Puente-Palazuelos, C. (2012). Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Applied Animal Behaviour Science, 140(3), 172–178. https://doi.org/10.1016/j.applanim.2012.06.002
Diggles, B. K. (2019). Review of some scientific issues related to crustacean welfare. ICES Journal of Marine Science, 76(1), 66–81. https://doi.org/10.1093/icesjms/fsy058
Dı́az, F., Sierra, E., Denisse Re, A., & Rodrı́guez, L. (2002). Behavioural thermoregulation and critical thermal limits of Macrobrachium acanthurus (Wiegman). Journal of Thermal Biology, 27(5), 423–428. https://doi.org/10.1016/S0306-4565(02)00011-6
dos Santos, D. B., Pontes, C. S., Campos, P. M. O., & De Fátima Arruda, M. (2015). Behavioral profile of Macrobrachium rosenbergii in mixed and monosex culture submitted to shelters of different colors. Acta Scientiarum. Biological Sciences, 37(3), 273. https://doi.org/10.4025/actascibiolsci.v37i3.24656
Duan, Y., Li, M., Sun, M., Wang, A., Chai, Y., Dong, J., Chen, F., Yu, Z., & Zhang, X. (2022). Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei. Sustainability, 14(22). https://doi.org/10.3390/su142215413
Duan, Y., Liu, Q., Wang, Y., Zhang, J., & Xiong, D. (2018b). Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish & Shellfish Immunology, 78, 279–288. https://doi.org/10.1016/j.fsi.2018.04.050
Duan, Y., Wang, Y., Zhang, J., & Xiong, D. (2018a). Elevated temperature disrupts the mucosal structure and induces an immune response in the intestine of whiteleg shrimp Litopenaeus vannamei (Boone, 1931) (Decapoda: Dendrobranchiata: Penaeidae). Journal of Crustacean Biology, 38(5), 635–640. https://doi.org/10.1093/jcbiol/ruy055
Duan, Y., Zhang, J., Dong, H., Wang, Y., Liu, Q., & Li, H. (2016b). Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish & Shellfish Immunology, 49, 91–99. https://doi.org/10.1016/j.fsi.2015.12.018
Duan, Y., Zhang, Y., Dong, H., & Zhang, J. (2016a). Effect of desiccation on oxidative stress and antioxidant response of the black tiger shrimp Penaeus monodon. Fish & Shellfish Immunology, 58, 10–17. https://doi.org/10.1016/j.fsi.2016.09.004
EFSA. (2005). Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to the aspects of the biology and welfare of animals used for experimental and other scientific purposes. EFSA Journal, 3(12), 292. https://doi.org/10.2903/j.efsa.2005.292
EFSA. (2013). EFSA aquatic ecotoxicology guidance incorporates a decade of scientific developments. EFSA. https://perma.cc/SGU7-ZY53
EGTOP. (2014). Final Report on Aquaculture (Part B). Expert Group for Technical Advice on Organic Production. https://perma.cc/QBJ5-YCXK
El-Sayed, A.-F. M. (2021). Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Reviews in Aquaculture, 13(1), 676–705. https://doi.org/10.1111/raq.12494
Estrada-Pérez, A., Ruiz-Velazco, J. M. J., Hernández-Llamas, A., Zavala-Leal, I., & Martínez-Cárdenas, L. (2016). Deterministic and stochastic models for analysis of partial harvesting strategies and improvement of intensive commercial production of whiteleg shrimp (Litopenaeus vannamei). Aquacultural Engineering, 70, 56–62. https://doi.org/10.1016/j.aquaeng.2015.11.003
Eswaran, S. (2021). Specific Pathogen Free (SPF) Shrimps in Aquaculture. In P. K. Pandey & J. Parhi (Eds.), Advances in Fisheries Biotechnology (pp. 465–470). Springer Nature. https://doi.org/10.1007/978-981-16-3215-0_27
European Commission (2023). Attitudes of Europeans towards animal welfare. Eurobarometer. European Commission. https://perma.cc/4PXV-P9CR
Evans, S. R., Finnie, M., & Manica, A. (2007). Shoaling preferences in decapod crustacea. Animal Behaviour, 74(6), 1691–1696. https://doi.org/10.1016/j.anbehav.2007.03.017
FAO. (2003). Health management and biosecurity maintenance in white shrimp (Penaeus vannamei) hatcheries in Latin America (450; FAO Fisheries Technical Paper). FAO. https://perma.cc/QD9J-866H
FAO. (2007). Improving Penaeus monodon hatchery practices. Manual based on experience in India (446; FAO Fisheries Technical Paper). FAO. https://perma.cc/UX8J-JLBL
FAO. (2009a). Penaeus vannamei. In cultured aquatic species fact sheets. https://perma.cc/DT32-95S6
FAO. (2009b). Macrobrachium rosenbergii. In cultured aquatic species fact sheets. https://perma.cc/6KJD-T6N7
FAO. (2023a). Global capture production. Fisheries and Aquaculture Division. https://perma.cc/6ERV-W2F5
FAO. (2023b). Global Aquaculture Production. Fisheries and Aquaculture Division. https://perma.cc/F3RX-8ZH9
FAO. (2023c). Penaeus monodon. In cultured aquatic species fact sheets. https://perma.cc/72G5-H63W
Farfante, I. P. (1969). Western Atlantic Shrimps of the Genus Penaeus. Fishery Bulletin, 67(3). https://perma.cc/HED7-QY8H
Farnsworth, K. D., & Elwood, R. W. (2023). Why it hurts: with freedom comes the biological need for pain. Animal Cognition, 26(4), 1259–1275. https://doi.org/10.1007/s10071-023-01773-2
Fast, A. W. (1992). Penaeid Growout Systems: An Overview. In A. W. Fast & L. J. Lester (Eds.), Marine Shrimp Culture: Principles and Practices (pp. 345–353). Elsevier. https://doi.org/10.1016/B978-0-444-88606-4.50020-5
Faulkes, Z. (2014). Invertebrate pain, and reporting on replications. https://perma.cc/M8JY-B7ZV
Fleckenstein, L. J., Kring, N. A., Tierney, T. W., Fisk, J. C., Lawson, B. C., & Ray, A. J. (2020). The effects of artificial substrate and stocking density on Pacific white shrimp (Litopenaeus vannamei) performance and water quality dynamics in high tunnel-based biofloc systems. Aquacultural Engineering, 90, 102093. https://doi.org/10.1016/j.aquaeng.2020.102093
Flick, G. J., & Kuhn, D. D. (2015). Shrimp out of water: Shipping live shrimp in waterless conditions. Global Seafood Alliance. https://perma.cc/9ARU-XBHR
Florida Shore Fishing. (2021). Live or Frozen Shrimp for Bait | Fishing from Florida Shores. https://perma.cc/VS6A-7V7U
Frías-Espericueta, M. G., Abad-Rosales, S., Nevárez-Velázquez, A. C., Osuna-López, I., Páez-Osuna, F., Lozano-Olvera, R., & Voltolina, D. (2008). Histological effects of a combination of heavy metals on Pacific white shrimp Litopenaeus vannamei juveniles. Aquatic Toxicology, 89(3), 152–157. https://doi.org/10.1016/j.aquatox.2008.06.010
Frías-Espericueta, M. G., Harfush-Melendez, M., & Páez-Osuna, F. (2000). Effects of Ammonia on Mortality and Feeding of Postlarvae Shrimp Litopenaeus vannamei. Bulletin of Environmental Contamination and Toxicology, 65(1), 98–103. https://doi.org/10.1007/s0012800100
Furtado, P. S., Fugimura, M. M. S., Monserrat, J. M., Souza, D. M., Garcia, L. de O., & Wasielesky, W. (2015). Acute effects of extreme pH and its influences on the survival and biochemical biomarkers of juvenile White Shrimp, Litopenaeus vannamei. Marine and Freshwater Behaviour and Physiology, 48(6), 417–429. https://doi.org/10.1080/10236244.2015.1086539
Gao, W., Tian, L., Huang, T., Yao, M., Hu, W., & Xu, Q. (2016). Effect of salinity on the growth performance, osmolarity and metabolism-related gene expression in white shrimp Litopenaeus vannamei. Aquaculture Reports, 4, 125–129. https://doi.org/10.1016/j.aqrep.2016.09.001
García-de la Parra, L. M., Bautista-Covarrubias, J. C., Rivera-de la Rosa, N., Betancourt-Lozano, M., & Guilhermino, L. (2006). Effects of methamidophos on acetylcholinesterase activity, behavior, and feeding rate of the white shrimp (Litopenaeus vannamei). Ecotoxicology and Environmental Safety, 65(3), 372–380. https://doi.org/10.1016/j.ecoenv.2005.09.001
González, R. A., Díaz, F., Licea, A., Denisse Re, A., Noemí Sánchez, L., & García-Esquivel, Z. (2010). Thermal preference, tolerance and oxygen consumption of adult white shrimp Litopenaeus vannamei (Boone) exposed to different acclimation temperatures. Journal of Thermal Biology, 35(5), 218–224. https://doi.org/10.1016/j.jtherbio.2010.05.004
Gooding, E. L., Kendrick, M. R., Brunson, J. F., Kingsley‐Smith, P. R., Fowler, A. E., Frischer, M. E., & Byers, J. E. (2020). Black gill increases the susceptibility of white shrimp, Penaeus setiferus (Linnaeus, 1767), to common estuarine predators. Journal of Experimental Marine Biology and Ecology, 524, 151284. https://doi.org/10.1016/j.jembe.2019.151284
Goodrick, B., Paterson, B., & Grauf, S. (1993). Live transport of prawns in sawdust: A comparison of wild and cultured species. In B. Goodrick, B. Paterson, & S. Grauf (Eds.), Transportation and postharvest handling of ocean caught prawns destined for live export (pp. 41–47). Report to the Fisheries Research and Development Corporation (Project 91/71). https://perma.cc/QFK6-6TVA
Gräslund, S., Holmström, K., & Wahlström, A. (2003). A field survey of chemicals and biological products used in shrimp farming. Marine Pollution Bulletin, 46(1), 81-90.
Grey, M., Forster, I., Dominy, W., Ako, H., & Giesen, A. F. (2009). Validation of a Feeding Stimulant Bioassay Using Fish Hydrolysates for the Pacific White Shrimp, Litopenaeus vannamei. Journal of the World Aquaculture Society, 40(4), 547–555. https://doi.org/10.1111/j.1749-7345.2009.00264.x
Guan, W., Nong, W., Wei, X., Zhu, M., & Mao, L. (2021). Impacts of a novel live shrimp (Litopenaeus vannamei) water-free transportation strategy on flesh quality: Insights through stress response and oxidation in lipids and proteins. Aquaculture, 533, 736168. https://doi.org/10.1016/j.aquaculture.2020.736168
Hamano, K., Miyoshi, T., Aue-umneoy, D., Srisapoome, P., Maeno, Y., & Tsutsui, I. (2015). Waterborne and cannibalism-mediated transmission of the Yellow head virus in Penaeus monodon. Aquaculture, 437, 161–166. https://doi.org/10.1016/j.aquaculture.2014.11.038
Han, S., Wang, B., Liu, M., Wang, M., Jiang, K., Liu, X., & Wang, L. (2018). Adaptation of the white shrimp Litopenaeus vannamei to gradual changes to a low-pH environment. Ecotoxicology and Environmental Safety, 149, 203–210. https://doi.org/10.1016/j.ecoenv.2017.11.052
Hardy, R. W. (2010). Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquaculture Research, 41(5), 770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
Hargreaves, J. A., & Boyd, C. E. (2022). Dissolved oxygen and aeration in penaeid shrimp aquaculture ponds. In V. Alday-Sanz (Ed.), The Shrimp Book II (pp. 206–266). 5m Books Ltd.
HATCH. (2019a). Harvesting Techniques. HATCH Global Shrimp Farm Technology Report. https://perma.cc/DJ93-PHK3
HATCH. (2019b). Welcome to the HATCH Global Shrimp Farm Technology Report. HATCH Global Shrimp Farm Technology Report. https://perma.cc/55HR-E3E3
HATCH. (2019c). Farm Challenges & Farmer Wish List. HATCH Global Shrimp Farm Technology Report. https://perma.cc/GYL3-XWHX
HATCH. (2019d). Infrastructure. HATCH Global Shrimp Farm Technology Report. https://perma.cc/3NGP-2F5L
HATCH. (2019e). Feed Management and Administration. HATCH Global Shrimp Farm Technology Report. https://perma.cc/3Z2R-JZF6
HATCH. (2019f). Biosecurity measures. HATCH Global Shrimp Farm Technology Report. https://perma.cc/5HSM-QT2K
Heal, R. D., Hasan, N. A., & Haque, M. M. (2021). Increasing disease burden and use of drugs and chemicals in Bangladesh shrimp aquaculture: A potential menace to human health. Marine Pollution Bulletin, 172, 112796. https://doi.org/10.1016/j.marpolbul.2021.112796
Hinchliffe, S., Butcher, A., & Rahman, M. M. (2018). The AMR problem: Demanding economies, biological margins, and co-producing alternative strategies. Palgrave Communications, 4(1), Article 1. https://doi.org/10.1057/s41599-018-0195-4
Hoang, T., Ho, H. C., Le, N. P. T., & Bui, T. H. H. (2020). Effects of high temperature on survival and feed consumption of banana shrimp Penaeus merguiensis. Aquaculture, 522, 735152. https://doi.org/10.1016/j.aquaculture.2020.735152
Hook, S. E., Doan, H., Gonzago, D., Musson, D., Du, J., Kookana, R., Sellars, M. J., & Kumar, A. (2018). The impacts of modern-use pesticides on shrimp aquaculture: An assessment for north eastern Australia. Ecotoxicology and Environmental Safety, 148, 770–780. https://doi.org/10.1016/j.ecoenv.2017.11.028
Howell, C., James, T., Yussof, R., Yazid, A., & Suni, S. (2013). Brunei project develops technology for large black tiger shrimp production, part 5. Global Seafood Alliance. https://perma.cc/3F6M-KB8H
Hsieh, H.-H., Weerathunga, V., Weerakkody, W. S., Huang, W.-J., Muller, F. L. L., Benfield, M. C., & Hung, C.-C. (2021). The effects of low pH on the taste and amino acid composition of tiger shrimp. Scientific Reports, 11(1), Article 1. https://doi.org/10.1038/s41598-021-00612-z
Huang, Z., Guan, W., Lyu, X., Chen, R., Wu, Y., Zheng, G., & Mao, L. (2023). Impacts of long-time transportation on whiteleg shrimp (Penaeus vannamei) muscle quality and underlying biochemical mechanisms. Journal of the Science of Food and Agriculture. https://doi.org/10.1002/jsfa.12841
Islam, G. M.R., Habib, M. R., Waid, J. L., Rahman, M. S., Kabir, J., Akter, S., & Jolly, Y. N. (2017). Heavy metal contamination of freshwater prawn (Macrobrachium rosenbergii) and prawn feed in Bangladesh: A market-based study to highlight probable health risks. Chemosphere, 170, 282–289. https://doi.org/10.1016/j.chemosphere.2016.11.163
Jia, X., Wang, F., Lu, Y., Zhang, D., & Dong, S. (2014). Immune responses of Litopenaeus vannamei to thermal stress: A comparative study of shrimp in freshwater and seawater conditions. Marine and Freshwater Behaviour and Physiology, 47(2), 79–92. https://doi.org/10.1080/10236244.2014.894349
Jiang, S., Zhou, F. L., Zeng, X. Y., Yang, Q. B., Huang, J. H., Yang, L. S., & Jiang, S. G. (2021). Effects of four factors on Penaeus monodon post-larvae cannibalism. Iranian Journal of Fisheries Sciences, 20(2), 547–557. https://perma.cc/WEF3-YFU5
Jones, A. B., Preston, N. P., & Dennison, W. C. (2002). The efficiency and condition of oysters and macroalgae used as biological filters of shrimp pond effluent. Aquaculture research, 33(1), 1-19. https://doi.org/10.1046/j.1355-557X.2001.00637.x
Jory, D. E., & Akiyama, D. (2006). Feed Management. In C. E. Boyd, D. E. Jory, & G. W. Chamberlain (Eds.), Operating Procedures for Shrimp Farming: Global Shrimp OP Survey Results and Recommendations (pp. 85–96). Global Aquaculture Alliance. https://perma.cc/QY5F-HXNQ
Kang, B. J., Okutsu, T., Tsutsui, N., Shinji, J., Bae, S.-H., & Wilder, M. N. (2014). Dynamics of Vitellogenin and Vitellogenesis-Inhibiting Hormone Levels in Adult and Subadult Whiteleg Shrimp, Litopenaeus vannamei: Relation to Molting and Eyestalk Ablation1. Biology of Reproduction, 90(1), 12, 1–10. https://doi.org/10.1095/biolreprod.113.112243
Kawamura, G., Bagarinao, T., Yong, A. S. K., Fen, T. C., & Lim, L. S. (2017). Shelter colour preference of the postlarvae of the giant freshwater prawn Macrobrachium rosenbergii. Fisheries Science, 83(2), 259–264. https://doi.org/10.1007/s12562-017-1062-8
Kawamura, G., Bagarinao, T., Yong, A. S. K., Jeganathan, I. M. X., & Lim, L.-S. (2016). Colour preference and colour vision of the larvae of the giant freshwater prawn Macrobrachium rosenbergii. Journal of Experimental Marine Biology and Ecology, 474, 67–72. https://doi.org/10.1016/j.jembe.2015.10.001
Kenyon, R. A., Loneragan, N. R., & Hughes, J. M. (1995). Habitat type and light affect sheltering behaviour of juvenile tiger prawns (Penaeus esculentus Haswell) and success rates of their fish predators. Journal of Experimental Marine Biology and Ecology, 192(1), 87–105. https://doi.org/10.1016/0022-0981(95)00064-X
Knipe, H., Temperton, B., Lange, A., Bass, D., & Tyler, C. R. (2020). Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Reviews in Aquaculture, 13(1), 324–352. https://doi.org/10.1111/raq.12477
Krause, J., Ruxton, G. D. (2002). Living in Groups. Oxford University Press.
Kring, N. A., Fleckenstein, L. J., Tierney, T. W., Fisk, J. C., Lawson, B. C., & Ray, A. J. (2023). The effects of stocking density and artifical substrate on production of pacific white shrimp Litopenaeus vannamei and water quality dynamics in greenhouse-based biofloc systems. Aquacultural Engineering, 101, 102322. https://doi.org/10.1016/j.aquaeng.2023.102322
Krummenauer, D., Peixoto, S., Cavalli, R. O., Poersch, L. H., & Wasielesky Jr, W. (2011). Superintensive Culture of White Shrimp, Litopenaeus vannamei, in a Biofloc Technology System in Southern Brazil at Different Stocking Densities. Journal of the World Aquaculture Society, 42(5), 726–733. https://doi.org/10.1111/j.1749-7345.2011.00507.x
Kubitza, F. (2017). The oft-overlooked water quality parameter: pH. Global Seafood Alliance. https://perma.cc/NGN7-SFDB
Kuhn, D., & Taylor, D. P. (2017). Waterless Shipment of Warm-Water Shrimp. Virginia Cooperative Extension. https://perma.cc/U56Q-HHYL
Kumlu, M., Kumlu, M., & Türkmen, S. (2010). Combined effects of temperature and salinity on critical thermal minima of pacific white shrimp Litopenaeus vannamei (Crustacea: Penaeidae). Journal of Thermal Biology, 35(6), 302–304. https://doi.org/10.1016/j.jtherbio.2010.06.008
Kungvankij, P., Chua, T., Pudadera, B., Corre, K. G., Borlongan, E., Alava, Tiro, L. B., Potestas, I. O., & Talean, G. A. (1986a). Feeds and Feeding (NACA Training Manual Series No. 2 Shrimp Culture: Pond Design, Operation and Management). FAO. https://perma.cc/3RFG-AQWX
Kungvankij, P., Chua, T., Pudadera, B., Corre, K. G., Borlongan, E., Alava, Tiro, L. B., Potestas, I. O., & Talean, G. A. (1986b). Water Quality Management (NACA Training Manual Series No. 2 Shrimp Culture: Pond Design, Operation and Management). FAO. https://perma.cc/FD5P-5ERL
Kungvankij, P., Chua, T., Pudadera, B., Corre, K. G., Borlongan, E., Alava, Tiro, L. B., Potestas, I. O., & Talean, G. A. (1986c). Pond preparation (NACA Training Manual Series No. 2 Shrimp Culture: Pond Design, Operation and Management). FAO. https://perma.cc/47TQ-43BD
Kuo, C. M., & Yang, Y. H. (1999). Hyperglycemic responses to cold shock in the freshwater giant prawn, Macrobrachium rosenbergii. Journal of Comparative Physiology B, 169(1), 49–54. https://doi.org/10.1007/s003600050192
Kutty, M.N. (1987). Site Selection For Aquaculture: Physical features of water. FAO. https://perma.cc/UP4F-LDZM
Lara, G., Hostins, B., Bezerra, A., Poersch, L., & Wasielesky, W. (2017). The effects of different feeding rates and re-feeding of Litopenaeus vannamei in a biofloc culture system. Aquacultural Engineering, 77, 20–26. https://doi.org/10.1016/j.aquaeng.2017.02.003
Laramore, S., Laramore, C. R., & Scarpa, J. (2001). Effect of Low Salinity on Growth and Survival of Postlarvae and Juvenile Litopenaeus vannamei. Journal of the World Aquaculture Society, 32(4), 385–392. https://doi.org/10.1111/j.1749-7345.2001.tb00464.x
Lazur, A. (2007). JIFSAN Good Aquacultural Practices Program. Growout Pond and Water Quality Management. Joint Institute for Food Safety and Applied Nutrition. https://perma.cc/LM7V-B37H
Le Moullac, G., & Haffner, P. (2000). Environmental factors affecting immune responses in Crustacea. Aquaculture, 191(1), 121–131. https://doi.org/10.1016/S0044-8486(00)00422-1
Li, N., Zhao, Y., & Yang, J. (2007). Impact of Waterborne Copper on the Structure of Gills and Hepatopancreas and Its Impact on the Content of Metallothionein in Juvenile Giant Freshwater Prawn Macrobrachium rosenbergii (Crustacea: Decapoda). Archives of Environmental Contamination and Toxicology, 52(1), 73–79. https://doi.org/10.1007/s00244-005-0214-5
Lin, C. K, & Drazba, L. (2006). Pond Water Quality. In C. E. Boyd, D. E. Jory, & G. W. Chamberlain (Eds.), Operating Procedures for Shrimp Farming: Global Shrimp OP Survey Results and Recommendations. Global Aquaculture Alliance. https://perma.cc/QY5F-HXNQ
Lin, Y.-C., & Chen, J.-C. (2001). Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. Journal of Experimental Marine Biology and Ecology, 259(1), 109–119. https://doi.org/10.1016/S0022-0981(01)00227-1
Liu, F., Li, S., Yu, Y., Sun, M., Xiang, J., & Li, F. (2020). Effects of ammonia stress on the hemocytes of the Pacific white shrimp Litopenaeus vannamei. Chemosphere, 239, 124759. https://doi.org/10.1016/j.chemosphere.2019.124759
Liu, H.-L., Yang, S.-P., Wang, C.-G., Chan, S.-M., Wang, W.-X., Feng, Z.-H., & Sun, C.-B. (2015). Effect of Air Exposure and Resubmersion on the Behavior and Oxidative Stress of Pacific White Shrimp Litopenaeus vannamei. North American Journal of Aquaculture, 77(1), 43–49. https://doi.org/10.1080/15222055.2014.955157
Lord, J. P., Moser, R. M., Buonocore, E. M., Sylvester, E. E., Morales, M. J., Granitz, A. P., Disipio, A., Blakely, E., O’Sullivan-Evangelista, S. L., Mateo, T. F., Chlebove, G. J., Carey, C. M., & Lucas, O. (2021). Dominance Hierarchies in Marine Invertebrates. The Biological Bulletin, 240(1), 2–15. https://doi.org/10.1086/712973
Lucas, C., Kirkwood, G., & Somers, I. (1979). An Assessment of the Stocks of the Banana Prawn Penaeus merguiensis in the Gulf of Carpentaria. Marine and Freshwater Research, 30(5), 639–652. https://doi.org/10.1071/mf9790639
Luchiari, A. C., Marques, A. O., & Freire, F. a. M. (2012). Effects of substrate colour preference on growth of the shrimp Litopenaeus vannamei (Boone, 1931) (Decapoda, Penaeoidea). Crustaceana, 85(7), 789–800. https://doi.org/10.1163/156854012X650232
Lucien-Brun, H. (2016a). Critical decisions for shrimp harvesting and packing, Part 1. Global Seafood Alliance. https://perma.cc/M5RF-4DXM
Lucien-Brun, H. (2016b). Critical decisions for shrimp harvesting and packing, Part 2. Global Seafood Alliance. https://perma.cc/6UAD-QZYZ
Lyle-Fritch, L. P., Romero-Beltrán, E., & Páez-Osuna, F. (2006). A survey on use of the chemical and biological products for shrimp farming in Sinaloa (NW Mexico). Aquacultural Engineering, 35(2), 135–146. https://doi.org/10.1016/j.aquaeng.2005.09.006
Madenjian, C. P., Rogers, G. L., & Fast, A. W. (1987). Predicting night time dissolved oxygen loss in prawn ponds of Hawaii: Part I. Evaluation of traditional methods. Aquacultural Engineering, 6(3), 191–208. https://doi.org/10.1016/0144-8609(87)90004-5
Magaña-Gallegos, E., Bautista-Bautista, M., González-Zuñiga, L. M., Arevalo, M., Cuzon, G., & Gaxiola, G. (2018). Does unilateral eyestalk ablation affect the quality of the larvae of the pink shrimp Farfantepenaeus brasiliensis (Letreille, 1817) (Decapoda: Dendrobranchiata: Penaeidae)? Journal of Crustacean Biology, 38(4), 401–406. https://doi.org/10.1093/jcbiol/ruy043
Mariappan, P., & Balasundaram, C. (2003). Sheltering behaviour of Macrobrachium nobilii (Henderson and Matthai, 1910). Acta Ethologica, 5(2), 89–94. https://doi.org/10.1007/s10211-002-0069-y
McGraw, W. J., Davis, D. A., Teichert-Coddington, D., & Rouse, D. B. (2002). Acclimation of Litopenaeus vannamei Postlarvae to Low Salinity: Influence of Age, Salinity Endpoint, and Rate of Salinity Reduction. Journal of the World Aquaculture Society, 33(1), 78–84. https://doi.org/10.1111/j.1749-7345.2002.tb00481.x
McGraw, W., Teichert-Coddington, D. R., Rouse, D. B., & Boyd, C. E. (2001). Higher minimum dissolved oxygen concentrations increase penaeid shrimp yields in earthen ponds. Aquaculture, 199(3), 311–321. https://doi.org/10.1016/S0044-8486(01)00530-0
McLean, E., Barrows, F. T., Craig, S. R., Alfrey, K., & Tran, L. (2020). Complete replacement of fishmeal by soybean and poultry meals in Pacific whiteleg shrimp feeds: Growth and tolerance to EMS/AHPND and WSSV challenge. Aquaculture, 527, 735383. https://doi.org/10.1016/j.aquaculture.2020.735383
Medina-Reyna, C. E. (2001). Growth and emigration of white shimp, Litopenaeus vannamei, in the Mar Muerto Lagoon, Southern Mexico. Naga, 24(3–4), 30–34. https://perma.cc/3RZ7-PPJH
Mellor, D. J., & Beausoleil, N. J. (2015). Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Animal Welfare, 24(3), 241–253. https://doi.org/10.7120/09627286.24.3.241
Mellor, D. J., Beausoleil, N. J., Littlewood, K. E., McLean, A. N., McGreevy, P. D., Jones, B., & Wilkins, C. (2020). The 2020 five domains model: including Human–Animal Interactions in assessments of animal welfare. Animals, 10(10), 1870. https://doi.org/10.3390/ani10101870
Meshram, S. J., Shingare, P. E., & Ingle, S. T. (2009). Studies on traditional methods of wild giant freshwater prawn seed collection and their potential impact on the aquatic ecosystem. Asian Fisheries Science, 22, 185–189. https://doi.org/10.33997/j.afs.2009.22.1.017
Minello, T. J., Zimmerman, R. J., & Martinez, E. X. (1989). Mortality of Young Brown Shrimp Penaeus aztecus in Estuarine Nurseries. Transactions of the American Fisheries Society, 118(6), 693–708. https://doi.org/10.1577/1548-8659(1989)118<0693:MOYBSP>2.3.CO;2
Moreno-Arias, A., López-Elías, J. A., Martínez-Córdova, L. R., Ramírez-Suárez, J. C., Carvallo-Ruiz, M. G., García-Sánchez, G., Lugo-Sánchez, M. E., & Miranda-Baeza, A. (2018). Effect of fishmeal replacement with a vegetable protein mixture on the amino acid and fatty acid profiles of diets, biofloc and shrimp cultured in BFT system. Aquaculture, 483, 53–62. https://doi.org/10.1016/j.aquaculture.2017.10.011
Muñoz, M., Cedeño, R., Rodrı́guez, J., van der Knaap, W. P. W., Mialhe, E., & Bachère, E. (2000). Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penaeus vannamei. Aquaculture, 191(1), 89–107. https://doi.org/10.1016/S0044-8486(00)00420-8
National Research Council. (2011). Nutrient Requirements of Fish and Shrimp Report in Brief. National Academies Press. https://perma.cc/26GK-45NG
Naylor, R. L., Hardy, R. W., Buschmann, A. H., Bush, S. R., Cao, L., Klinger, D. H., Little, D. C., Lubchenco, J., Shumway, S. E., & Troell, M. (2021). A 20-year retrospective review of global aquaculture. Nature, 591(7851), Article 7851. https://doi.org/10.1038/s41586-021-03308-6
New, M. (2002). Farming freshwater prawns. A manual for the culture of the giant river prawn (Macrobrachium rosenbergii) (428; FAO Fisheries Technical Paper). FAO. https://perma.cc/8KSC-SHWG
Nga, B. T., Lürling, M., Peeters, E. T. H. M., Roijackers, R., Scheffer, M., & Nghia, T. T. (2005). Chemical and physical effects of crowding on growth and survival of Penaeus monodon Fabricius post-larvae. Aquaculture, 246(1), 455–465. https://doi.org/10.1016/j.aquaculture.2005.02.026
Nonwachai, T., Purivirojku, W., Chuchird, N., & Limsuwan, C. (2011). Effects of Dissolved Oxygen Levels on Growth, Survival and Immune Response of Juvenile Pacific White Shrimp Litopenaeus vannamei. Journal of Fisheries and Environment, 35(3), Article 3. https://perma.cc/L6TJ-AM39
Noor-Hamid, S., Fortes, R. D., & Parado-Estepa, F. (1994). Effect of pH and ammonia on survival and growth of the early larval stages of Penaeus monodon Fabricius. Aquaculture, 125(1), 67–72. https://doi.org/10.1016/0044-8486(94)90283-6
Nunes, A. J. P., Dalen, L. L., Leonardi, G., & Burri, L. (2022). Developing sustainable, cost-effective and high-performance shrimp feed formulations containing low fish meal levels. Aquaculture Reports, 27, 101422. https://doi.org/10.1016/j.aqrep.2022.101422
Nunes, A. J. P., Sá, M. V. C., Andriola-Neto, F. F., & Lemos, D. (2006). Behavioral response to selected feed attractants and stimulants in Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 260(1), 244–254. https://doi.org/10.1016/j.aquaculture.2006.06.027
Oddsson, G. V. (2020). A Definition of Aquaculture Intensity Based on Production Functions—The Aquaculture Production Intensity Scale (APIS). Water, 12(3), Article 3. https://doi.org/10.3390/w12030765
Ogle, J. T. (1992). A review of the current (1992) state of our knowledge concerning reproduction in open thelycum Penaeid shrimp with emphasis on Penaeus vannamei. Invertebrate Reproduction & Development, 22(1–3), 267–274. https://doi.org/10.1080/07924259.1992.9672279
Ohs, C. L., Grabe, S. W., & Creswell, R. L. (2006). The Utilization of a Fish Pump for Harvesting Shrimp from Tanks and Ponds: FA123/FA123, 2/2006. EDIS, 2006(8), Article 8. https://doi.org/10.32473/edis-fa123-2006
Ohs, C. L., Grabe, S. W., & Creswell, R. L. (2007). The Utilization of a Fish Pump for Harvesting Shrimp from Tanks and Ponds. The Fish Site. https://perma.cc/NU95-DCEV
Olin, P. G., & Fast, A. W. (1992). Penaeid Pl Harvest, Transport, Acclimation and Stocking. In A. W. Fast & L. J. Lester (Eds.), Marine Shrimp Culture (pp. 301–320). Elsevier. https://doi.org/10.1016/B978-0-444-88606-4.50018-7
Otwell, S., Garrido, L., Garrido, V., & Benner, R. (2001). Farm-raised shrimp: Good aquacultural practices for product quality and safety. Florida Sea Grant College Program. https://perma.cc/V5ML-XQG6
Páez-Osuna, F. (2001). The Environmental Impact of Shrimp Aquaculture: Causes, Effects, and Mitigating Alternatives. Environmental Management, 28(1), 131–140. https://doi.org/10.1007/s002670010212
Pan, L.-Q., Hu, F.-W., Jing, F.-T., & Liu, H.-J. (2008). The effect of different acclimation temperatures on the prophenoloxidase system and other defence parameters in Litopenaeus vannamei. Fish & Shellfish Immunology, 25(1), 137–142. https://doi.org/10.1016/j.fsi.2008.03.016
Panutrakul, S., & Senanan, W. (2021). Abundance of introduced Pacific whiteleg shrimp Penaeus vannamei (Boone, 1931) along the east coast of Thailand. Aquatic Invasions, 16(4), 750–770. https://doi.org/10.3391/ai.2021.16.4.10
Pedrazzani, A. S., Cozer, N., Quintiliano, M. H., Tavares, C. P. dos S., da Silva, U. de A. T., & Ostrensky, A. (2023). Non-Invasive Methods for Assessing the Welfare of Farmed White-Leg Shrimp (Penaeus vannamei). Animals, 13(5), Article 5. https://doi.org/10.3390/ani13050807
Penn, J. W., Caputi, N., & Melville-Smith, R. (2001). Crustacean Fisheries. In J. H. Steele (Ed.), Encyclopedia of Ocean Sciences (Second Edition) (pp. 699–707). Academic Press. doi.org/10.1016/B978-012374473-9.00453-7
Perkins, K. (2021). Can Aquatic Invertebrates within Public Aquaria Fit the Five Domain Welfare Model? Journal of Applied Animal Ethics Research, 3(2), 181–204. https://doi.org/10.1163/25889567-bja10025
Persyn, H., & Aungst, R. (2006). Nursery. In C. E. Boyd, D. E. Jory, & G. W. Chamberlain (Eds.), Operating Procedures For Shrimp Farming (pp. 42–51). Global Aquaculture Alliance. https://perma.cc/QY5F-HXNQ
Piana, E., Gautier, D., & Cooper, R. (2018). Evaluating stunning methods in tropical shrimp aquaculture. Global Seafood Alliance. https://perma.cc/GT6K-T7LR
Pontes, C. S., Arruda, M. de F., Augusto de Lara Menezes, A., & Pereira de Lima, P. (2006). Daily activity pattern of the marine shrimp Litopenaeus vannamei (Boone 1931) juveniles under laboratory conditions. Aquaculture Research, 37(10), 1001–1006. https://doi.org/10.1111/j.1365-2109.2006.01519.x
Pontes, C. S., Arruda, M. de F., Santana, V. G. da S., & Santos, D. B. dos. (2020). Animal performance and welfare of giant freshwater prawn (Macrobrachium rosenbergii) subjected to feed restriction. R. Bras. Zootec., 49. https://doi.org/10.37496/rbz4920190128
Prangnell, D. I., Lupatsch, I., Treece, G. D., & Samocha, T. M. (2019). Chapter 2—Shrimp Biology. In T. M. Samocha (Ed.), Sustainable Biofloc Systems for Marine Shrimp (pp. 19–27). Academic Press. https://doi.org/10.1016/B978-0-12-818040-2.00002-2
Primavera, J. (1989). Broodstock of sugpo, (Penaeus monodon Fabricius) (4th ed.). Aquaculture Department, Southeast Asian Fisheries Development Center. https://perma.cc/X3VL-YA6V
Puri, S., & Faulkes, Z. (2010). Do Decapod Crustaceans Have Nociceptors for Extreme pH? PLOS ONE, 5(4), e10244. https://doi.org/10.1371/journal.pone.0010244
Qian, D., Xu, C., Chen, C., Qin, J. G., Chen, L., & Li, E. (2020). Toxic effect of chronic waterborne copper exposure on growth, immunity, anti-oxidative capacity and gut microbiota of Pacific white shrimp Litopenaeus vannamei. Fish & Shellfish Immunology, 100, 445–455. https://doi.org/10.1016/j.fsi.2020.03.018
Quackenbush, L. S. (2001). Yolk Synthesis in the Marine Shrimp, Penaeus vannamei. American Zoologist, 41(3), 458–464. https://doi.org/10.1093/icb/41.3.458
Reis, J., Novriadi, R., Swanepoel, A., Jingping, G., Rhodes, M., & Davis, D. A. (2020). Optimizing feed automation: Improving timer-feeders and on demand systems in semi-intensive pond culture of shrimp Litopenaeus vannamei. Aquaculture, 519, 734759. https://doi.org/10.1016/j.aquaculture.2019.734759
Reis, J., Quintero, H. E., & Roy, L. (2022). Feeding practices for automated systems in commercial shrimp production. In V. Alday-Sanz (Ed.), The Shrimp Book II (pp. 357–373). 5m Books Ltd.
Ren, X., Wang, Q., Shao, H., Xu, Y., Liu, P., & Li, J. (2021). Effects of Low Temperature on Shrimp and Crab Physiology, Behavior, and Growth: A Review. Frontiers in Marine Science, 8. https://doi.org/10.3389/fmars.2021.746177
Rico, A., Phu, T. M., Satapornvanit, K., Min, J., Shahabuddin, A. M., Henriksson, P. J. G., Murray, F. J., Little, D. C., Dalsgaard, A., & Van den Brink, P. J. (2013). Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture, 412–413, 231–243. https://doi.org/10.1016/j.aquaculture.2013.07.028
Rivera-Velásquez, G., Soto, L. A., Salgado Ugarte, I. H., & Naranjo, E. J. (2008). Growth, mortality and migratory pattern of white shrimp (Litopenaeus vannamei, Crustacea, Penaeidae) in the Carretas-Pereyra coastal lagoon system, Mexico. Revista de Biología Tropical, 56(2), Article 2. https://doi.org/10.15517/rbt.v56i2.5605
Robertson, C. (Ed.). (2006). Australian Prawn Farming Manual: Health Management for profit. The State of Queensland, Department of Primary Industries and Fisheries. https://perma.cc/K7RY-93XZ
Romano, N., & Zeng, C. (2017). Cannibalism of Decapod Crustaceans and Implications for Their Aquaculture: A Review of its Prevalence, Influencing Factors, and Mitigating Methods. Reviews in Fisheries Science & Aquaculture, 25(1), 42–69. https://doi.org/10.1080/23308249.2016.1221379
Roy, L. A., Davis, D. A., Saoud, I. P., Boyd, C. A., Pine, H. J., & Boyd, C. E. (2010). Shrimp culture in inland low salinity waters. Reviews in Aquaculture, 2(4), 191–208. https://doi.org/10.1111/j.1753-5131.2010.01036.x
RSPCA Australia. (2016). Humane killing and processing of crustaceans for human consumption. RSPCA Australia. https://perma.cc/R688-3PZK
Sainz-Hernández, J. C., Racotta, I. S., Dumas, S., & Hernández-López, J. (2008). Effect of unilateral and bilateral eyestalk ablation in Litopenaeus vannamei male and female on several metabolic and immunologic variables. Aquaculture, 283(1), 188–193. https://doi.org/10.1016/j.aquaculture.2008.07.002
Salin, K. R., & Jayasree-Vadhyar, K. (2001). Effect of different chilling rates for cold anaesthetization of Penaeus monodon (Fabricius) on the survival, duration and sensory quality under live storage in chilled sawdust. Aquaculture Research, 32(2), 145–155. https://doi.org/10.1046/j.1365-2109.2001.00542.x
Salini, J. P., Blaber, S. J. M., & Brewer, D. T. (1990). Diets of piscivorous fishes in a tropical Australian estuary, with special reference to predation on penaeid prawns. Marine Biology, 105(3), 363–374. https://doi.org/10.1007/BF01316307
Santos, A. D. A., López-Olmeda, J. F., Sánchez-Vázquez, F. J., & Fortes-Silva, R. (2016). Synchronization to light and mealtime of the circadian rhythms of self-feeding behavior and locomotor activity of white shrimps (Litopenaeus vannamei). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 199, 54–61. https://doi.org/10.1016/j.cbpa.2016.05.001
Saputra, H. K., Hamka, M. S., Kurniaji, A., Susanti, L., Firman, S. W., Dwiarto, A., & Alam, H. S. (2022). The technology of shrimp larvae transportation: Ecophysiology and bioeconomy effects on high stocking density shrimp Litopenaeus vannamei. E3S Web of Conferences, 348, 00038. https://doi.org/10.1051/e3sconf/202234800038
Sasikumar, G., & Vadhyar, J. (1998). Effect of packing density and habitat material on the survival and duration of Penaeus indicus post larvae during oxygen-packed transportation. Tenth Kerala Science Congress. https://perma.cc/RN52-TN7Q
Schroeder, J. P., Gärtner, A., Waller, U., & Hanel, R. (2010). The toxicity of ozone-produced oxidants to the Pacific white shrimp Litopenaeus vannamei. Aquaculture, 305(1–4), 6–11. https://doi.org/10.1016/j.aquaculture.2010.03.030
Schveitzer, R., Arantes, R., Baloi, M. F., Costódio, P. F. S., Arana, L. V., Seiffert, W. Q., & Andreatta, E. R. (2013b). Use of artificial substrates in the culture of Litopenaeus vannamei (Biofloc System) at different stocking densities: Effects on microbial activity, water quality and production rates. Aquacultural Engineering, 54, 93–103. https://doi.org/10.1016/j.aquaeng.2012.12.003
Shinji, J., Kang, B. J., Okutsu, T., Banzai, K., Ohira, T., Tsutsui, N., & Wilder, M. N. (2012). Changes in crustacean hyperglycemic hormones in Pacific whiteleg shrimp Litopenaeus vannamei subjected to air-exposure and low-salinity stresses. Fisheries Science, 78(4), 833–840. https://doi.org/10.1007/s12562-012-0514-4
Shinji, J., Nohara, S., Yagi, N., & Wilder, M. (2019). Bio-economic analysis of super-intensive closed shrimp farming and improvement of management plans: A case study in Japan. Fisheries Science, 85(6), 1055–1065. https://doi.org/10.1007/s12562-019-01357-5
Shrimp Insights. (2020). SPF L. vannamei broodstock report (Shrimp Insights Report Series). Shrimp Insights. https://perma.cc/9698-TTUC
Shrimp Welfare Project. (2022). India Scoping Report. Shrimp Welfare Project. https://perma.cc/V2NT-GH52
Sinclair, M., Lee, N. Y. P., Hötzel, M. J., De Luna, M. C. T., Sharma, A., Idris, M., Derkley, T., Li, C., Islam, M. A., Iyasere, O. S., Navarro, G., Ahmed, A. A., Khruapradab, C., Curry, M., Burns, G. L., & Marchant, J. (2022). International perceptions of animals and the importance of their welfare. Frontiers in Animal Science, 3. https://doi.org/10.3389/fanim.2022.960379
Skudlarek, J. G., Coyle, S. D., Bright, L. A., & Tidwell, J. H. (2011). Effect of Holding and Packing Conditions on Hemolymph Parameters of Freshwater Prawns, Macrobrachium rosenbergii, during Simulated Waterless Transport. Journal of the World Aquaculture Society, 42(5), 603–617. https://doi.org/10.1111/j.1749-7345.2011.00508.x
Smith, D. m., Tabrett, S. j., Barclay, M. c., & Irvin, S. j. (2005). The efficacy of ingredients included in shrimp feeds to stimulate intake. Aquaculture Nutrition, 11(4), 263–272. https://doi.org/10.1111/j.1365-2095.2005.00349.x
Sofyani, A., & Sambhu, C. (2020). Role of artificial substrates on growth performance of Pacific white shrimp, Litopenaeus vannamei (Boone, 1931) in semi-arid lands. Indian Journal of Geo Marine Sciences, 49(04), 576–580. https://perma.cc/65GS-2SG6
Stentiford, G. D., Neil, D. M., Peeler, E. J., Shields, J. D., Small, H. J., Flegel, T. W., Vlak, J. M., Jones, B., Morado, F., Moss, S., Lotz, J., Bartholomay, L., Behringer, D. C., Hauton, C., & Lightner, D. V. (2012). Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. Journal of Invertebrate Pathology, 110(2), 141–157. https://doi.org/10.1016/j.jip.2012.03.013
Suantika, G., Situmorang, M. L., Kurniawan, J. B., Pratiwi, S. A., Aditiawati, P., Astuti, D. I., Azizah, F. F. N., Djohan, Y. A., Zuhri, U., & Simatupang, T. M. (2018). Development of a zero water discharge (ZWD)—Recirculating aquaculture system (RAS) hybrid system for super intensive white shrimp (Litopenaeus vannamei) culture under low salinity conditions and its industrial trial in commercial shrimp urban farming in Gresik, East Java, Indonesia. Aquacultural Engineering, 82, 12–24. https://doi.org/10.1016/j.aquaeng.2018.04.002
Suresh, A. V., Kumaraguru vasagam, K. P., & Nates, S. (2011). Attractability and palatability of protein ingredients of aquatic and terrestrial animal origin, and their practical value for blue shrimp, Litopenaeus stylirostris fed diets formulated with high levels of poultry byproduct meal. Aquaculture, 319(1), 132–140. https://doi.org/10.1016/j.aquaculture.2011.06.039
Tani, M., & Kuramoto, T. (1998). Cool-sensitive Neurons in the Ventral Nerve Cord of Crustaceans. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 119(3), 845–852. https://doi.org/10.1016/S1095-6433(98)01025-3
Taylor, J., Vinatea, L., Ozorio, R., Schuweitzer, R., & Andreatta, E. R. (2004). Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture, 233(1), 173–179. https://doi.org/10.1016/j.aquaculture.2003.09.034
Teixeira, A. P. G., & Guerrelhas, A. C. B. (2014). What size are your postlarvae? Global Seafood Alliance. https://perma.cc/5M7Z-K546
Tendencia, E. A., Bosma, R. H., & Verreth, J. A. J. (2011). White spot syndrome virus (WSSV) risk factors associated with shrimp farming practices in polyculture and monoculture farms in the Philippines. Aquaculture, 311(1), 87–93. https://doi.org/10.1016/j.aquaculture.2010.11.039
Terazaki, M., Tharnbuppa, P., & Nakayama, Y. (1980). Eradication of predatory fishes in shrimp farms by utilization of Thai tea seed. Aquaculture, 19(3), 235–242. https://doi.org/10.1016/0044-8486(80)90047-2
Thakur, D. P., & Lin, C. K. (2003). Water quality and nutrient budget in closed shrimp (Penaeus monodon) culture systems. Aquacultural Engineering, 27(3), 159–176. https://doi.org/10.1016/S0144-8609(02)00055-9
Tierney, T. W., Fleckenstein, L. J., & Ray, A. J. (2020). The effects of density and artificial substrate on intensive shrimp Litopenaeus vannamei nursery production. Aquacultural Engineering, 89, 102063. https://doi.org/10.1016/j.aquaeng.2020.102063
Tran, L.H., Fitzsimmons, K.M., & Lightner, D.V. (2014). Tilapia could enhance water conditions, help control EMS. Global Seafood Alliance. https://perma.cc/T8CY-9EK3
Treece, G. D., & Fox, J. M. (1993). Design, Operation and Training Manual for an Intensive Culture Shrimp Hatchery. Texas A&M Uniersity Sea Grant College Programme. https://perma.cc/9M88-CXD6
Ullman, C., Rhodes, M. A., & Allen Davis, D. (2019a). Feed management and the use of automatic feeders in the pond production of Pacific white shrimp Litopenaeus vannamei. Aquaculture, 498, 44–49. https://doi.org/10.1016/j.aquaculture.2018.08.040
Ullman, C., Rhodes, M., Hanson, T., Cline, D., & Davis, D. A. (2019b). Effects of Four Different Feeding Techniques on the Pond Culture of Pacific White Shrimp, Litopenaeus vannamei. Journal of the World Aquaculture Society, 50(1), 54–64. https://doi.org/10.1111/jwas.12531
Vaiyapuri, M., Pailla, S., Rao Badireddy, M., Pillai, D., Chandragiri Nagarajarao, R., & Prasad Mothadaka, M. (2021). Antimicrobial resistance in Vibrios of shrimp aquaculture: Incidence, identification schemes, drivers and mitigation measures. Aquaculture Research, 52(7), 2923–2941. https://doi.org/10.1111/are.15142
Valenti, W. C., Daniels, W. H., New, M. B., & Correia, E. S. (2009b). Hatchery Systems and Management. In Freshwater Prawns (pp. 55–85). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781444314649.ch5
Valenti, W. C., New, M. B., Salin, K. R., & Ye, J. (2009a). Grow-Out Systems – Monoculture. In M. B. New, W. C. Valenti, J. H. Tidwell, L. R. D’Abramo, & M. N. Kutty (Eds.), Freshwater Prawns: Biology and Farming (pp. 154–179). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781444314649.ch9
Vazquez, L., Alpuche, J., Maldonado, G., Agundis, C., Pereyra-Morales, A., & Zenteno, E. (2009). Review: Immunity mechanisms in crustaceans. Innate Immunity, 15(3), 179–188. https://doi.org/10.1177/1753425909102876
Venkateswarlu, V., Seshaiah, P., Arun, P., & Behra, P. (2019). A study on water quality parameters in shrimp L. vannamei semi-intensive grow out culture farms in coastal districts of Andhra Pradesh, India. International Journal of Fisheries and Aquatic Studies, 7(4), 394–399. https://perma.cc/BU5Y-LKXX
Villalón, J. R. (1991). Practical manual for semi-intensive commercial production of marine shrimp. Texas A&M University Sea Grant College Program. https://perma.cc/7N9D-NPUA
Waldhorn, D. R., & Autric, E. (2023). Shrimp production: Understanding the scope of the problem. OSF Preprints. https://doi.org/doi.org/10.31219/osf.io/b8n3t
Walker, P. J., & Mohan, C. V. (2009). Viral disease emergence in shrimp aquaculture: Origins, impact and the effectiveness of health management strategies. Reviews in Aquaculture, 1(2), 125–154. https://doi.org/10.1111/j.1753-5131.2009.01007.x
Wang, Z., Qu, Y., Yan, M., Li, J., Zou, J., & Fan, L. (2019). Physiological Responses of Pacific White Shrimp Litopenaeus vannamei to Temperature Fluctuation in Low-Salinity Water. Frontiers in Physiology, 10. https://doi.org/10.3389/fphys.2019.01025
Wang, Z., Zhou, J., Li, J., Zou, J., & Fan, L. (2020). The immune defense response of Pacific white shrimp (Litopenaeus vannamei) to temperature fluctuation. Fish & Shellfish Immunology, 103, 103–110. https://doi.org/10.1016/j.fsi.2020.04.053
Weineck, K., Ray, A. J., Fleckenstein, L. J., Medley, M., Dzubuk, N., Piana, E., & Cooper, R. L. (2018). Physiological Changes as a Measure of Crustacean Welfare under Different Standardized Stunning Techniques: Cooling and Electroshock. Animals : An Open Access Journal from MDPI, 8(9), 158. https://doi.org/10.3390/ani8090158
Wickins, J. F., & Lee, D. O. (2002). Crustacean Farming: Ranching and Culture. John Wiley & Sons. http://doi.org/10.1002/9780470995082
WOAH. (2022). Aquatic Animal Health Code. WOAH - World Organisation for Animal Health. https://perma.cc/8GPW-2W3L
Wolfe, J. M., Breinholt, J. W., Crandall, K. A., Lemmon, A. R., Lemmon, E. M., Timm, L. E., Siddall, M. E., & Bracken-Grissom, H. D. (2019). A phylogenomic framework, evolutionary timeline and genomic resources for comparative studies of decapod crustaceans. Proceedings of the Royal Society B: Biological Sciences, 286(1901), 20190079. https://doi.org/10.1098/rspb.2019.0079
Wu, J. L., Namikoshi, A., Nishizawa, T., Mushiake, K., Teruya, K., & Muroga, K. (2001). Effects of shrimp density on transmission of penaeid acute viremia in Penaeus japonicus by cannibalism and the waterborne route. Diseases of Aquatic Organisms, 47(2), 129–135. https://doi.org/10.3354/dao047129
Xu, D., Wu, J., Sun, L., Qin, X., Fan, X., & Zheng, X. (2021). Combined stress of acute cold exposure and waterless duration at low temperature induces mortality of shrimp Litopenaeus vannamei through injuring antioxidative and immunological response in hepatopancreas tissue. Journal of Thermal Biology, 100, 103080. https://doi.org/10.1016/j.jtherbio.2021.103080
Xu, W.-J., Pan, L.-Q., Zhao, D.-H., & Huang, J. (2012). Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture, 350–353, 147–153. https://doi.org/10.1016/j.aquaculture.2012.04.003
Xue, S., Ding, J., Li, J., Jiang, Z., Fang, J., Zhao, F., & Mao, Y. (2021). Effects of live, artificial and mixed feeds on the growth and energy budget of Penaeus vannamei. Aquaculture Reports, 19, 100634. https://doi.org/10.1016/j.aqrep.2021.100634
Ye, L., Jiang, S., Zhu, X., Yang, Q., Wen, W., & Wu, K. (2009). Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture, 290(1), 140–144. https://doi.org/10.1016/j.aquaculture.2009.01.028
You, X., Su, Y., Mao, Y., Liu, M., Wang, J., Zhang, M., & Wu, C. (2010). Effect of high water temperature on mortality, immune response and viral replication of WSSV-infected Marsupenaeus japonicus juveniles and adults. Aquaculture, 305(1), 133–137. https://doi.org/10.1016/j.aquaculture.2010.04.024
Yu, Q., Xie, J., Huang, M., Chen, C., Qian, D., Qin, J. G., Chen, L., Jia, Y., & Li, E. (2020). Growth and health responses to a long-term pH stress in Pacific white shrimp Litopenaeus vannamei. Aquaculture Reports, 16, 100280. https://doi.org/10.1016/j.aqrep.2020.100280
Yu, R., Leung, P., & Bienfang, P. (2009). Modeling partial harvesting in intensive shrimp culture: A network-flow approach. European Journal of Operational Research, 193(1), 262–271. https://doi.org/10.1016/j.ejor.2007.10.031
Zacarias, S., Fegan, D., Wangsoontorn, S., Yamuen, N., Limakom, T., Carboni, S., Davie, A., Metselaar, M., Little, D. C., & Shinn, A. P. (2021). Increased robustness of postlarvae and juveniles from non-ablated Pacific whiteleg shrimp, Penaeus vannamei, broodstock post-challenged with pathogenic isolates of Vibrio parahaemolyticus (VpAHPND) and white spot disease (WSD). Aquaculture, 532, 736033. https://doi.org/10.1016/j.aquaculture.2020.736033
Zhang, B., Lin, W., Huang, J., Wang, Y., & Xu, R. (2010). Effects of artificial substrates on the growth, survival and spatial distribution of Litopenaeus vannamei in the intensive culture condition. Iranian Journal of Fisheries Sciences, 9(2), 293–304. https://perma.cc/2HWW-3TJ6
Zhang, P., Zhang, X., Li, J., & Huang, G. (2006). The effects of body weight, temperature, salinity, pH, light intensity and feeding condition on lethal DO levels of whiteleg shrimp, Litopenaeus vannamei (Boone, 1931). Aquaculture, 256(1), 579–587. https://doi.org/10.1016/j.aquaculture.2006.02.020
Zhang, W., Ma, S., Li, X., Guo, Y., Ge, H., Huang, D., Chen, F., Wu, Y., & Lei, K. (2022). Nutrition and shrimp health. In V. Alday-Sanz (Ed.), The Shrimp Book II (pp. 392–426). 5m Books Ltd.
Zhao, M., Yao, D., Li, S., Zhang, Y., & Aweya, J. J. (2020). Effects of ammonia on shrimp physiology and immunity: A review. Reviews in Aquaculture, 12(4), 2194–2211. https://doi.org/10.1111/raq.12429
Zhou, Y., Huang, M., Ge, J., & Davis, D. A. (2022). Mineral requirements of shrimp. In V. Alday-Sanz (Ed.), The Shrimp Book II (pp. 374–391). 5m Books Ltd.
Notes
-
There are several larval stages, namely nauplii, myses, and zoeae, but we group them under the term ‘larvae’ for simplicity. ↩
-
Raceways are channel-shaped with a continuous current of water flowing through them. ↩
-
Historically, P. monodon broodstock has been harder to domesticate, so farming of this species may rely more on wild-caught broodstock. Some domestication of this species has happened and may increase in the future (as reported by Robins McIntosh at the Global Shrimp Forum, 2022). ↩
-
We consulted an expert who wished to remain anonymous here, who confirmed that “most” farmed shrimp come from hatcheries, without knowing a clear percentage. Another said that all P. vannamei shrimp come from domesticated broodstock, while another was skeptical that any farms currently use wild-caught postlarvae. ↩
-
Nurseries give a more closely monitored environment during the postlarval stage, when shrimp are more sensitive to water quality and disease, resulting in higher survival and providing more slack in water quality when shrimp are put into ongrowing ponds (Persyn & Aungst, 2006, p. 42). ↩
-
We note that providing live feed for shrimp would also likely have welfare repercussions for the individuals given as shrimp feed, but this is beyond the scope of this report. ↩
-
Kona Bay broodstock company advertises their ‘Kona Bay Strength’ shrimp, reported to be “specially selected for disease tolerance in open systems to [two viruses and to bacteria].” ↩
-
We found no consistent estimations about the relative prevalence of each listed disease but many sources report that White Spot Syndrome and Acute Hepatopancreatic Necrosis Disease (often referred to as Early Mortality Syndrome; EMS) have most negatively impacted industry production (Asche et al., 2021, p.5; Benchmark Insights, 2019, p. 53; Walker & Mohan, 2009, p. 126) ↩
-
The exception is for breeders and Macrobrachium shrimp (Wickins & Lee, 2002, pp. 164–166), where shelters (e.g., PVC pipes) and substrates are used to discourage juveniles’ typical aggressive behavior, though individuals still compete over shelters (Balasundaram et al., 2004; New, 2002, pp. 75–77; Wickins & Lee, 2002, p. 168). ↩
-
This study was conducted with a density of 510 shrimp/m2 and shrimp choices may differ at lower densities ↩
-
This assumption about the color of plastic liners is based on pictures in articles about shrimp farms (e.g., HATCH, 2019d; Howell et al., 2013; Robertson et al., 2006, p. 21). ↩
-
There are indirect welfare threats caused by feed management, such as diminished water quality caused by overfeeding—this is covered in the Water quality section. ↩
-
Kang et al. (2014) found that gonad-inhibiting hormone was not reduced after eyestalk ablation, but ovarian maturity was still induced. They note that other peptides that inhibit maturation are situated in the eyestalk. ↩
-
Elena Piana (personal communication, July 12, 2021) for example, said that many farmers do not have access to freshwater and as such would find it easier to use marine water, which is already on the farm when shrimp are harvested, while additional infrastructure would be needed to obtain freshwater. ↩